Immunonutrition for acute respiratory distress syndrome (ARDS) in adults
- PMID: 30677127
- PMCID: PMC6353063
- DOI: 10.1002/14651858.CD012041.pub2
Immunonutrition for acute respiratory distress syndrome (ARDS) in adults
Abstract
Background: Acute respiratory distress syndrome (ARDS) is an overwhelming systemic inflammatory process associated with significant morbidity and mortality. Pharmacotherapies that moderate inflammation in ARDS are lacking. Several trials have evaluated the effects of pharmaconutrients, given as part of a feeding formula or as a nutritional supplement, on clinical outcomes in critical illness and ARDS.
Objectives: To systematically review and critically appraise available evidence on the effects of immunonutrition compared to standard non-immunonutrition formula feeding on mechanically ventilated adults (aged 18 years or older) with acute respiratory distress syndrome (ARDS).
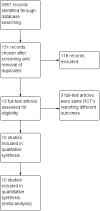
Search methods: We searched MEDLINE, Embase, CENTRAL, conference proceedings, and trial registries for appropriate studies up to 25 April 2018. We checked the references from published studies and reviews on this topic for potentially eligible studies.
Selection criteria: We included all randomized controlled trials (RCTs) and quasi-randomized controlled trials comparing immunonutrition versus a control or placebo nutritional formula in adults (aged 18 years or older) with ARDS, as defined by the Berlin definition of ARDS or, for older studies, by the American-European Consensus Criteria for both ARDS and acute lung injury.
Data collection and analysis: Two review authors independently assessed the quality of studies and extracted data from the included trials. We sought additional information from study authors. We performed statistical analysis according to Cochrane methodological standards. Our primary outcome was all-cause mortality. Secondary outcomes included intensive care unit (ICU) length of stay, ventilator days, indices of oxygenation, cardiac adverse events, gastrointestinal adverse events, and total number of adverse events. We used GRADE to assess the quality of evidence for each outcome.
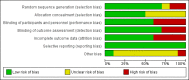
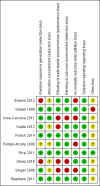
Main results: We identified 10 randomized controlled trials with 1015 participants. All studies compared an enteral formula or additional supplemental omega-3 fatty acids (i.e. eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA)), gamma-linolenic acid (GLA), and antioxidants. We assessed some of the included studies as having high risk of bias due to methodological shortcomings. Studies were heterogenous in nature and varied in several ways, including type and duration of interventions given, calorific targets, and reported outcomes. All studies reported mortality. For the primary outcome, study authors reported no differences in all-cause mortality (longest period reported) with the use of an immunonutrition enteral formula or additional supplements of omega-3 fatty acids and antioxidants (risk ratio (RR) 0.79, 95% confidence interval (CI) 0.59 to 1.07; participants = 1015; studies = 10; low-quality evidence).For secondary outcomes, we are uncertain whether immunonutrition with omega-3 fatty acids and antioxidants reduces ICU length of stay (mean difference (MD) -3.09 days. 95% CI -5.19 to -0.99; participants = 639; studies = 8; very low-quality evidence) and ventilator days (MD -2.24 days, 95% CI -3.77 to -0.71; participants = 581; studies = 7; very low-quality evidence). We are also uncertain whether omega-3 fatty acids and antioxidants improve oxygenation, defined as ratio of partial pressure of arterial oxygen (PaO₂) to fraction of inspired oxygen (FiO₂), at day 4 (MD 39 mmHg, 95% CI 10.75 to 67.02; participants = 676; studies = 8), or whether they increase adverse events such as cardiac events (RR 0.87, 95% CI 0.09 to 8.46; participants = 339; studies = 3; very low-quality evidence), gastrointestinal events (RR 1.11, 95% CI 0.71 to 1.75; participants = 427; studies = 4; very low-quality evidence), or total adverse events (RR 0.91, 95% CI 0.67 to 1.23; participants = 517; studies = 5; very low-quality evidence).
Authors' conclusions: This meta-analysis of 10 studies of varying quality examined effects of omega-3 fatty acids and/or antioxidants in adults with ARDS. This intervention may produce little or no difference in all-cause mortality between groups. We are uncertain whether immunonutrition with omega-3 fatty acids and antioxidants improves the duration of ventilator days and ICU length of stay or oxygenation at day 4 due to the very low quality of evidence. Adverse events associated with immunonutrition are also uncertain, as confidence intervals include the potential for increased cardiac, gastrointestinal, and total adverse events.
Conflict of interest statement
AD: none known.
RC: none known.
VB: none known.
MPWG: leads the Xtreme‐Everest Oxygen Research Consortium, which has received unrestricted grant funding from BOC Medical (Linde Group), Eli Lilly Critical Care, Smith Medical, Deltex Medical, London Clinic, and Rolex. None of this funding has been paid to Dr. Grocott; rather, all funds have been paid directly to the home institutions of researchers within the Consortium. Dr. Grocott has also received honoraria for speaking (not related to this review) and/or travel expenses from Baxter, Fresenius‐Kabi, BOC Medical (Linde Group), and Eli Lilly Critical Care (all before 2010). MG serves on the medical advisory board of Sphere Medical Ltd and receives funding paid via University of Southampton. MG has received unrestricted study funding from Sphere Medical Ltd and Pharmacosmos Ltd.
PCC: In the last three years, PCC has received advisory and/or speaking honoraria from Fresenius‐Kabi (2016 to 2018), Baxter Healthcare (2016), Abbott Nutrition (2017), and Danone/Nutricia (2016, 2017) ‐ sellers of parenteral and enteral feeds, some of which were used in the studies included in this review, and from Pronova BioPharma/BASF AS (2016, 2017, 2018) and Smartfish (2016, 2017, 2018) ‐ sellers of products containing omega‐3 fatty acids.
Figures
























Update of
References
References to studies included in this review
Elamin 2012 {published data only}
Gadek 1999 {published data only}
-
- Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma‐linoleic acid, and antioxidants in patients with acute respiratory distress syndrome. Critical Care Medicine 1999;27(8):1409‐20. [PUBMED: 10470743] - PubMed
-
- Nelson JL, DeMichele SJ, Pacht ER, Wennberg AK, Enteral Nutrition in ARDS Study Group. Effect of enteral feeding with eicosapentaenoic acid, gamma‐linolenic acid, and antioxidants on antioxidant status in patients with acute respiratory distress syndrome. Journal of Parenteral and Enteral Nutrition 2003;27(2):98‐104. [PUBMED: 12665164] - PubMed
-
- Pacht ER, DeMichele SJ, Nelson JL, Hart J, Wennberg AK, Gadek JE. Enteral nutrition with eicosapentaenoic acid, gamma‐linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Critical Care Medicine 2003;31(2):491‐500. [PUBMED: 12576957] - PubMed
Grau‐Camona 2011 {published data only}
-
- Grau‐Carmona T, Morán‐García V, García‐de‐Lorenzo A, Heras‐de‐la‐Calle G, Quesada‐Bellver B, López‐Martínez J, et al. Effect of an enteral diet enriched with eicosapentaenoic acid, gamma‐linolenic acid and anti‐oxidants on the outcome of mechanically ventilated, critically ill, septic patients. Clinical Nutrition 2011;30(5):578‐84. [PUBMED: 21474219] - PubMed
Gupta 2012 {published data only}
Parish 2014 {published data only}
Pontes‐Arruda 2006 {published data only}
-
- Pontes‐Arruda A, Aragão AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma‐linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Critical Care Medicine 2006;34(9):2325‐33. [PUBMED: 16850002] - PubMed
Rice 2011 {published data only}
Shirai 2015 {published data only}
Singer 2006 {published data only}
-
- Singer P, Theilla M, Fisher H, Gibstein L, Grozovski E, Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma‐linolenic acid in ventilated patients with acute lung injury. Critical Care Medicine 2006;34(4):1033‐8. [PUBMED: 16484911] - PubMed
-
- Theilla M, Singer P, Cohen J, Dekeyser F. A diet enriched in eicosapentaenoic acid, gamma‐linolenic acid and antioxidants in the prevention of new pressure ulcer formation in critically ill patients with acute lung injury: a randomized, prospective, controlled study. Clinical Nutrition 2007;26(6):752‐7. [PUBMED: 17933438] - PubMed
Stapleton 2011 {published data only}
Additional references
Andrews 2011
-
- Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011;342:d1542. [PUBMED: 21415104] - PubMed
Ashbaugh 1967
-
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2(7511):319‐23. [PUBMED: 4143721] - PubMed
Beale 1999
-
- Beale RJ, Bryg DJ, Bihari DJ. Immunonutrition in the critically ill: a systematic review of clinical outcome. Critical Care Medicine 1999;27(12):2799‐805. [PUBMED: 10628629] - PubMed
Bernard 1994
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American‐European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine 1994;149:818‐24. [PUBMED: 7509706] - PubMed
Calder 2007
-
- Calder PC. Immunonutrition in surgical and critically ill patients. British Journal of Nutrition 2007;98(Suppl 1):S133‐9. [PUBMED: 17922951] - PubMed
Dee 2011
-
- Dee BM, Bruno JJ, Lal LS, Canada TW. Effects of immune‐enhancing enteral nutrition on mortality and oxygenation in acute lung injury and acute respiratory distress syndrome: a meta‐analysis. Hospital Pharmacy 2011;46(1):33‐40.
Dushianthan 2011
-
- Dushianthan A, Grocott MP, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgraduate Medical Journal 2011;87(1031):612‐22. [PUBMED: 21642654] - PubMed
Egger 1997
Gajic 2011
-
- Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. American Journal of Respiratory and Critical Care Medicine 2011;183(4):462‐70. [PUBMED: 20802164] - PMC - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 6 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [PUBMED: 16345038] - PubMed
Herridge 2011
-
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz‐Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. New England Journal of Medicine 2011;364(14):1293‐304. [PUBMED: 21470008] - PubMed
Heyland 2001
-
- Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001;286(8):944‐53. [PUBMED: 11509059] - PubMed
Heyland 2005
-
- Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Medicine 2005;31(3):327‐37. [PUBMED: 15605227] - PubMed
Heyland 2013
-
- Heyland DK, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. A randomized trial of glutamine and antioxidants in critically ill patients. New England Journal of Medicine 2013;368(16):1489‐97. [PUBMED: 23594003] - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hozo 2005
Lang 2002
-
- Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant‐antioxidant balance in acute lung injury. Chest 2002;122(Suppl 6):314S‐20S. [PUBMED: 12475808] - PubMed
Li 2015
-
- Li C, Bo L, Liu W, Lu X, Jin F. Enteral immunomodulatory diet (omega‐3 fatty acid, γ‐linolenic acid and antioxidant supplementation) for acute lung injury and acute respiratory distress syndrome: an updated systematic review and meta‐analysis. Nutrients 2015;7(7):5572‐85. [PUBMED: 26184293] - PMC - PubMed
Mancuso 1997a
-
- Mancuso P, Whelan J, DeMichele SJ, Snider CC, Guszcza JA, Karlstad MD. Dietary fish oil and fish and borage oil suppress intrapulmonary proinflammatory eicosanoid biosynthesis and attenuate pulmonary neutrophil accumulation in endotoxic rats. Critical Care Medicine 1997;25(7):1198‐206. [PUBMED: 9233748] - PubMed
Mancuso 1997b
-
- Mancuso P, Whelan J, DeMichele SJ, Snider CC, Guszcza JA, Claycombe KJ, et al. Effects of eicosapentaenoic and gamma‐linolenic acid on lung permeability and alveolar macrophage eicosanoid synthesis in endotoxic rats. Critical Care Medicine 1997;25(3):523‐32. [PUBMED: 9118672] - PubMed
Matthay 2011
McClave 2016
-
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society of Parenteral and Enteral Nutrition (A.S.P.E.N). Journal of Parenteral and Enteral Nutrition 2016;40(2):159‐211. [PUBMED: 26773077] - PubMed
Metnitz 1999
-
- Metnitz PG, Bartens C, Fischer M, Fridrich P, Steltzer H, Druml W. Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Medicine 1999;25(2):180‐5. [PUBMED: 10193545] - PubMed
Mizock 2010
-
- Mizock BA. Immunonutrition and critical illness: an update. Nutrition 2010;26:701‐7. [PUBMED: 20381315 ] - PubMed
Murray 1995
-
- Murray MJ, Kumar M, Gregory TJ, Banks PL, Tazelaar HD, DeMichele SJ. Select dietary fatty acids attenuate cardiopulmonary dysfunction during acute lung injury in pigs. American Journal of Physiology 1995;269(6 Pt 2):H2090‐9. [PUBMED: 8594921] - PubMed
Newsholme 1985
-
- Newsholme EA, Crabtree B, Ardawi MS. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Quarterly Journal of Experimental Physiology 1985;70(4):473‐89. [PUBMED: 3909197] - PubMed
Novak 2002
-
- Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Critical Care Medicine 2002;30(9):2022‐9. [MEDLINE: ] - PubMed
Pontes‐Arruda 2008
-
- Pontes‐Arruda A, Demichele S, Seth A, Singer P. The use of an inflammation‐modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta‐analysis of outcome data. Journal of Parenteral and Enteral Nutrition 2008;32(6):596‐605. [PUBMED: 18974237] - PubMed
Popovic 2007
-
- Popovic PJ, Zeh HJ 3rd, Ochoa JB. Arginine and immunity. Journal of Nutrition 2007;137(6 Suppl 2):1681S‐6S. [PUBMED: 17513447] - PubMed
Ranieri 2012
-
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307(23):2526‐33. [PUBMED: 22797452] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sane 2000
-
- Sane S, Baba M, Kusano C, Shirao K, Andoh T, Kamada T, et al. Eicosapentaenoic acid reduces pulmonary edema in endotoxemic rats. Journal of Surgical Research 2000;93(1):21‐7. [PUBMED: 10945939] - PubMed
Schmidt 2004
-
- Schmidt R, Luboeinski T, Markart P, Ruppert C, Daum C, Grimminger F, et al. Alveolar antioxidant status in patients with acute respiratory distress syndrome. European Respiratory Journal 2004;24(6):994‐9. [PUBMED: 15572544] - PubMed
Soares 2014
-
- Soares AD, Costa KA, Wanner SP, Santos RG, Fernandes SO, Martins FS, et al. Dietary glutamine prevents the loss of intestinal barrier function and attenuates the increase in core body temperature induced by acute heat exposure. British Journal of Nutrition 2014;112(10):1601‐10. [PUBMED: 25322775] - PubMed
van Zanten 2015
Walkey 2012
Wang 2014
Zhu 2014
-
- Zhu D, Zhang Y, Li S, Gan L, Feng H, Nie W. Enteral omega‐3 fatty acid supplementation in adult patients with acute respiratory distress syndrome: a systematic review of randomized controlled trials with meta‐analysis and trial sequential analysis. Intensive Care Medicine 2014;40(4):504‐12. [PUBMED: 24556914] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

