Malformations of Cerebral Cortex Development: Molecules and Mechanisms
- PMID: 30677308
- PMCID: PMC6938687
- DOI: 10.1146/annurev-pathmechdis-012418-012927
Malformations of Cerebral Cortex Development: Molecules and Mechanisms
Abstract
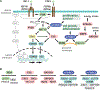
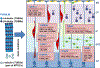
Malformations of cortical development encompass heterogeneous groups of structural brain anomalies associated with complex neurodevelopmental disorders and diverse genetic and nongenetic etiologies. Recent progress in understanding the genetic basis of brain malformations has been driven by extraordinary advances in DNA sequencing technologies. For example, somatic mosaic mutations that activate mammalian target of rapamycin signaling in cortical progenitor cells during development are now recognized as the cause of hemimegalencephaly and some types of focal cortical dysplasia. In addition, research on brain development has begun to reveal the cellular and molecular bases of cortical gyrification and axon pathway formation, providing better understanding of disorders involving these processes. New neuroimaging techniques with improved resolution have enhanced our ability to characterize subtle malformations, such as those associated with intellectual disability and autism. In this review, we broadly discuss cortical malformations and focus on several for which genetic etiologies have elucidated pathogenesis.
Keywords: focal cortical dysplasia; hemimegalencephaly; hippocampal dysgenesis; lissencephaly; microcephaly; polymicrogyria.
Figures


References
-
- Bystron I, Blakemore C, Rakic P. 2008. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 9:110–132 - PubMed
-
- Kuzniecky RI. 1994. Magnetic resonance imaging in developmental disorders of the cerebral cortex. Epilepsia 35 Suppl 6:S44–56 - PubMed
-
- Leventer RJ, Phelan EM, Coleman LT, et al. 1999. Clinical and imaging features of cortical malformations in childhood. Neurology 53:715–722 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

