Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population
- PMID: 30677750
- PMCID: PMC6589826
- DOI: 10.1159/000496484
Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population
Abstract
Background: To determine the association of vancomycin with acute kidney injury (AKI) in relation to its serum concentration value and to examine the risk of AKI in patients treated with vancomycin when compared with a matched cohort of patients receiving non-glycopeptide antibiotics (linezolid/daptomycin).
Methods: From a cohort of > 3 million US veterans with baseline estimated glomerular filtration rate ≥60 mL/min/1.73 m2, we identified 33,527 patients who received either intravenous vancomycin (n = 22,057) or non-glycopeptide antibiotics (linezolid/daptomycin, n = 11,470). We examined the association of the serum trough vancomycin level recorded within the first 48 h of administration with subsequent AKI in all patients treated with vancomycin and association of vancomycin vs. non-glycopeptide antibiotics use with the risk of incident AKI.
Results: The overall multivariable adjusted ORs of AKI stages 1, 2, and 3 in patients on vancomycin vs. non-glycopeptides were 1.1 (1.1-1.2), 1.2 (1-1.4), and 1.4 (1.1-1.7), respectively. When examined in strata divided by vancomycin trough level, the odds of AKI were similar or lower in patients receiving vancomycin compared to non-glycopeptide antibiotics as long as serum vancomycin levels were ≤20 mg/L. However, in patients with serum vancomycin levels > 20 mg/L, the ORs of AKI stages 1, 2, and 3 in patients on vancomycin vs. non-glycopeptide antibiotics were 1.5 (1.4-1.7), 1.9 (1.5-2.3), and 2.7 (2-3.5), respectively.
Conclusions: Vancomycin use is associated with a higher risk of AKI when serum levels exceed > 20 mg/L.
Keywords: Outcomes; Acute kidney injury; Daptomycon; Linezolid; Vancomycin.
© 2019 S. Karger AG, Basel.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
None of the authors has relevant conflicts of interest.
Figures


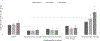

References
-
- Liu C, et al., Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis, 2011. 52(3): p. 285–92. - PubMed
-
- Levine DP, Vancomycin: a history. Clin Infect Dis, 2006. 42 Suppl 1: p. S5–12. - PubMed
-
- Tattevin P, et al., Efficacy and quality of antibacterial generic products approved for human use: a systematic review. Clin Infect Dis, 2014. 58(4): p. 458–69. - PubMed

