Ganoderma lucidum Polysaccharides Prevent Palmitic Acid-Evoked Apoptosis and Autophagy in Intestinal Porcine Epithelial Cell Line via Restoration of Mitochondrial Function and Regulation of MAPK and AMPK/Akt/mTOR Signaling Pathway
- PMID: 30678035
- PMCID: PMC6387170
- DOI: 10.3390/ijms20030478
Ganoderma lucidum Polysaccharides Prevent Palmitic Acid-Evoked Apoptosis and Autophagy in Intestinal Porcine Epithelial Cell Line via Restoration of Mitochondrial Function and Regulation of MAPK and AMPK/Akt/mTOR Signaling Pathway
Abstract
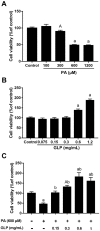
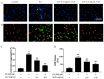
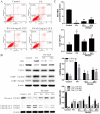
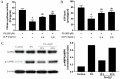
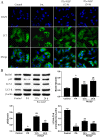
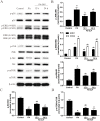
Ganoderma lucidum polysaccharide (GLP) extracted from Ganoderma lucidum (Leyss. ex Fr.) Karst, a traditional Chinese medicine, is a biologically active substance reported to possess anti-oxidative, anti-apoptotic, and neurological protection. However, it is unknown whether GLP have any protective effect against high-fat constituents-induced epithelial cell injury. The aim of this study was to investigate the protection and molecular mechanism of GLP on injury induced by palmitic acid (PA) in the intestinal porcine epithelial cell line (IPEC-J2). First, we tested whether the treatment of GLP attenuate PA-induced IPEC-J2 cell death. GLP markedly blocked PA-caused cytotoxicity and apoptosis in IPEC-J2 cells. Moreover, GLP recovered the decreased mitochondrial function and inhibited activation of caspase-dependent apoptotic pathway. Interestingly, PA promoted cell apoptosis and autophagy through stimulation of phosphorylation of mitogen-activated protein kinases (MAPKs), AMP-activated protein kinase (AMPK), and inhibition of phosphorylation of Akt and mammalian target of rapamycin (mTOR), which was reversed by GLP. Taken together, this study revealed a protective effect of GLP against PA-evoked IPEC-J2 cell death through anti-apoptotic and anti-autophagic properties.
Keywords: Ganoderma lucidum; apoptosis; autophagy; mitochondria; polysaccharides.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Akbari P., Fink-Gremmels J., Willems R.H.A.M., Difilippo E., Schols M.H.C., Garssen J., Braber S. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: Insight into the role of structure and size. Eur. J. Nutr. 2017;56:1919–1930. doi: 10.1007/s00394-016-1234-9. - DOI - PMC - PubMed
-
- Nguyen H.T.T., Dalmasso G., Müller S., Carrière J., Seibold F., Darfeuille-Michaud A. Crohn’s disease-associated adherent invasive escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy. Gastroenterology. 2014;146:508–519. doi: 10.1053/j.gastro.2013.10.021. - DOI - PubMed
-
- Elkahoui S., Bartley G.E., Yokoyama W.H., Friedman M. Dietary supplementation of potato peel powders prepared from conventional and organic russet and non-organic gold and red potatoes reduces weight gain in mice on a high-fat diet. J. Agric. Food Chem. 2018;66:6064–6072. doi: 10.1021/acs.jafc.8b01987. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 31501484/National Natural Science Foundation of China
- 2016JJ3081/Hunan Provincial Natural Science Foundation of China
- 2017GC03/Hunan Agricultural Science and Technology Innovation Team Project
- 2018QN27/Hunan Agricultural Science and Technology Innovation Team Project
- 2018ZD04/Hunan Agricultural Science and Technology Innovation Team Project
LinkOut - more resources
Full Text Sources
Miscellaneous

