Sphingolipid Metabolism: New Insight into Ceramide-Induced Lipotoxicity in Muscle Cells
- PMID: 30678043
- PMCID: PMC6387241
- DOI: 10.3390/ijms20030479
Sphingolipid Metabolism: New Insight into Ceramide-Induced Lipotoxicity in Muscle Cells
Abstract
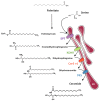
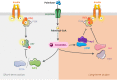
Insulin-resistance is a characteristic feature of type 2 diabetes (T2D) and plays a major role in the pathogenesis of this disease. Skeletal muscles are quantitatively the biggest glucose users in response to insulin and are considered as main targets in development of insulin-resistance. It is now clear that circulating fatty acids (FA), which are highly increased in T2D, play a major role in the development of muscle insulin-resistance. In healthy individuals, excess FA are stored as lipid droplets in adipocytes. In situations like obesity and T2D, FA from lipolysis and food are in excess and eventually accumulate in peripheral tissues. High plasma concentrations of FA are generally associated with increased risk of developing diabetes. Indeed, ectopic fat accumulation is associated with insulin-resistance; this is called lipotoxicity. However, FA themselves are not involved in insulin-resistance, but rather some of their metabolic derivatives, such as ceramides. Ceramides, which are synthetized de novo from saturated FA like palmitate, have been demonstrated to play a critical role in the deterioration of insulin sensitivity in muscle cells. This review describes the latest progress involving ceramides as major players in the development of muscle insulin-resistance through the targeting of selective actors of the insulin signaling pathway.
Keywords: DAG; ceramide; diabetes; insulin; sphingolipids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

