Understanding the Mechanism of Antidepressant-Related Sexual Dysfunction: Inhibition of Tyrosine Hydroxylase in Dopaminergic Neurons after Treatment with Paroxetine but Not with Agomelatine in Male Rats
- PMID: 30678046
- PMCID: PMC6406445
- DOI: 10.3390/jcm8020133
Understanding the Mechanism of Antidepressant-Related Sexual Dysfunction: Inhibition of Tyrosine Hydroxylase in Dopaminergic Neurons after Treatment with Paroxetine but Not with Agomelatine in Male Rats
Abstract
Antidepressant-related sexual dysfunction is a frequent adverse event caused by serotonergic activation that intensely affects quality of life and adherence in depressed patients. The dopamine system has multiple effects promoting sexual behavior, but no studies have been carried out to confirm dopaminergic changes involved in animal models after antidepressant use.
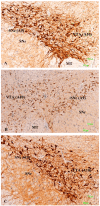
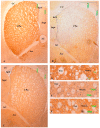
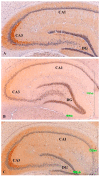
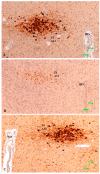
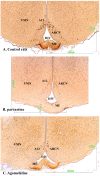
Methods: The sexual behavior-related dopaminergic system in the rat was studied by comparing two different antidepressants and placebo for 28 days. The antidepressants used were paroxetine (a serotonergic antidepressant that causes highly frequent sexual dysfunction in humans) and agomelatine (a non-serotonergic antidepressant without associated sexual dysfunction). The tyrosine hydroxylase immunoreactivity (THI) in the substantia nigra pars compacta, the ventral tegmental area, the zona incerta, and the hypothalamic arcuate nucleus, as well as the dopaminergic projections to the striatum, hippocampus, cortex, and median eminence were analyzed.
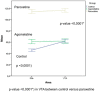
Results: The THI decreased significantly in the substantia nigra and ventral tegmental area after treatment with paroxetine, and the labeling was reduced drastically in the zona incerta and mediobasal hypothalamus. The immunoreactive axons in the target regions (striatum, cortex, hippocampus, and median eminence) almost disappeared only in the paroxetine-treated rats. Conversely, after treatment with agomelatine, a moderate reduction in immunoreactivity in the substantia nigra was found without appreciable modifications in the ventral tegmental area, zona incerta, and mediobasal hypothalamus. Nevertheless, no sexual or copulatory behavior was observed in any of the experimental or control groups.
Conclusion: Paroxetine but not agomelatine was associated with important decreased activity in dopaminergic areas such as the substantia nigra and ventral tegmental areas that could be associated with sexual performance impairment in humans after antidepressant treatment.
Keywords: agomelatine; dopaminergic system; immunohistochemical study; male rats; paroxetine; sexual dysfunction.
Conflict of interest statement
Dr. Montejo has received consultancy fees or honoraria/research grants in the last 5 years from Eli Lilly, Forum Pharmaceuticals, Rovi, Servier, Lundbeck, Otsuka, Janssen Cilag, Pfizer, Roche, Instituto de Salud Carlos III, and the Junta de Castilla y León. The rests of the authors declare no conflict of interest.
Figures

References
-
- Steinbusch H.W.M. Serotonin immunoreactive neurons and their projections in the CNS. In: Björklung A., Hökfelt T., Kuhar M.J., editors. Handbook of Chemical Anatomy: Classical Transmitters and Transmitter Receptors in the CNS Part II. 1st ed. Volume 3. Elsevier; New York, NY, USA: 1984. pp. 68–125.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

