Mother or Father: Who Is in the Front Line? Mechanisms Underlying the Non-Genomic Transmission of Obesity/Diabetes via the Maternal or the Paternal Line
- PMID: 30678214
- PMCID: PMC6413176
- DOI: 10.3390/nu11020233
Mother or Father: Who Is in the Front Line? Mechanisms Underlying the Non-Genomic Transmission of Obesity/Diabetes via the Maternal or the Paternal Line
Abstract
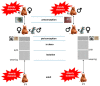
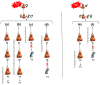
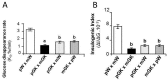
Extensive epidemiological and experimental evidence have shown that exposure to an adverse intrauterine environment as observed in offspring of pregnancies complicated by obesity or diabetes, can program susceptibility to metabolic, endocrine and cardiovascular disorders later in life. Although most studies have concentrated on the maternal environment, it is also becoming evident that paternal exposure to obesity or diabetes can result in the later development of metabolic disorders in the offspring. Such programmed effects might not be limited to the first directly exposed generation, but could be transmitted to subsequent generations. This suggests the existence of mechanisms by which metabolic changes in parental phenotype are transmissible to offspring. The mechanisms which underpin the transmission of the programmed effects across generations are still unclear. However, epigenetic regulation of transcription has emerged as a strong candidate for mediating the heritability of metabolic diseases. Here, we review the most relevant evidence from human and animal studies showing transmission of programming effects of obesity or diabetes across generations, and the current mechanisms underlying either maternal or paternal influences on the metabolic status of offspring.
Keywords: DOHaD; diabetes; germ cell epigenome; maternal and paternal metabolic imprinting; obesity; sncRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Voight B.F., Scott L.J., Steinthorsdottir V., Morris A.P., Dina C., Welch R.P., Zeggini E., Huth C., Aulchenko Y.S., Thorleifsson G., et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010;42:579–589. doi: 10.1038/ng.609. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

