20-Hydroxy-3-Oxolupan-28-Oic Acid Attenuates Inflammatory Responses by Regulating PI3K⁻Akt and MAPKs Signaling Pathways in LPS-Stimulated RAW264.7 Macrophages
- PMID: 30678231
- PMCID: PMC6385096
- DOI: 10.3390/molecules24030386
20-Hydroxy-3-Oxolupan-28-Oic Acid Attenuates Inflammatory Responses by Regulating PI3K⁻Akt and MAPKs Signaling Pathways in LPS-Stimulated RAW264.7 Macrophages
Abstract
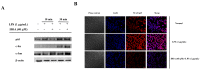
20-Hydroxy-3-oxolupan-28-oic acid (HOA), a lupane-type triterpene, was obtained from the leaves of Mahonia bealei, which is described in the Chinese Pharmacopeia as a remedy for inflammation and related diseases. The anti-inflammatory mechanisms of HOA, however, have not yet been fully elucidated. Therefore, the objective of this study was to characterize the molecular mechanisms of HOA in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. HOA suppressed the release of nitric oxide (NO), pro-inflammatory cytokine tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6) in LPS-stimulated RAW264.7 macrophages without affecting cell viability. Quantitative real-time reverse-transcription polymerase chain reaction (RT-qPCR) analysis indicated that HOA also suppressed the gene expression of inducible NO synthase (iNOS), TNF-α, and IL-6. Further analyses demonstrated that HOA inhibited the phosphorylation of upstream signaling molecules, including p85, PDK1, Akt, IκBα, ERK, and JNK, as well as the nuclear translocation of nuclear factor κB (NF-κB) p65. Interestingly, HOA had no effect on the LPS-induced nuclear translocation of activator protein 1 (AP-1). Taken together, these results suggest that HOA inhibits the production of cytokine by downregulating iNOS, TNF-α, and IL-6 gene expression via the downregulation of phosphatidylinositol 3-kinase (PI3K)/Akt and mitogen-activated protein kinases (MAPKs), and the inhibition of NF-κB activation. Our findings indicate that HOA could potentially be used as an anti-inflammatory agent for medical use.
Keywords: NF-κB; inflammation; lupane-type triterpene; macrophage; nitric oxide.
Conflict of interest statement
The authors claim no conflict of interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

