Discovery of New Cyclopentaquinoline Analogues as Multifunctional Agents for the Treatment of Alzheimer's Disease
- PMID: 30678364
- PMCID: PMC6386991
- DOI: 10.3390/ijms20030498
Discovery of New Cyclopentaquinoline Analogues as Multifunctional Agents for the Treatment of Alzheimer's Disease
Abstract
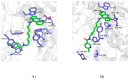
Here we report the two-step synthesis of 8 new cyclopentaquinoline derivatives as modifications of the tetrahydroacridine structure. Next, the biological assessment of each of them was performed. Based on the obtained results we identified 6-chloro-N-[2-(2,3-dihydro-1H-cyclopenta[b]quinolin-9-ylamino)-hexyl]]-nicotinamide hydrochloride (3e) as the most promising compound with inhibitory potencies against EeAChE and EqBuChE in the low nanomolar level 67 and 153 nM, respectively. Moreover, 3e compound is non-hepatotoxic, able to inhibit amyloid beta aggregation, and shows a mix-type of cholinesterase's inhibition. The mixed type of inhibition of the compound was confirmed by molecular modeling. Then, yeast three-hybrid (Y3H) technology was used to confirm the known ligand-receptor interactions. New derivatives do not show antioxidant activity (confirmed by the use of two different tests). A pKa assay method was developed to identify the basic physicochemical properties of 3e compound. A LogP assay confirmed that 3e compound fulfills Lipinsky's rule of five.
Keywords: Alzheimer’s disease; acetylcholinesterase inhibitors; beta amyloid; molecular modeling; yeast three-hybrid technology (Y3H) test.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
New Tetrahydroacridine Hybrids with Dichlorobenzoic Acid Moiety Demonstrating Multifunctional Potential for the Treatment of Alzheimer's Disease.Int J Mol Sci. 2020 May 26;21(11):3765. doi: 10.3390/ijms21113765. Int J Mol Sci. 2020. PMID: 32466601 Free PMC article.
-
Design, synthesis and biological evaluation of novel deoxyvasicinone-indole as multi-target agents for Alzheimer's disease.Bioorg Med Chem Lett. 2021 Oct 1;49:128212. doi: 10.1016/j.bmcl.2021.128212. Epub 2021 Jun 19. Bioorg Med Chem Lett. 2021. PMID: 34153471
-
Amiridine-piperazine hybrids as cholinesterase inhibitors and potential multitarget agents for Alzheimer's disease treatment.Bioorg Chem. 2021 Jul;112:104974. doi: 10.1016/j.bioorg.2021.104974. Epub 2021 May 21. Bioorg Chem. 2021. PMID: 34029971
-
Discovery of new phenyl sulfonyl-pyrimidine carboxylate derivatives as the potential multi-target drugs with effective anti-Alzheimer's action: Design, synthesis, crystal structure and in-vitro biological evaluation.Eur J Med Chem. 2021 Apr 5;215:113224. doi: 10.1016/j.ejmech.2021.113224. Epub 2021 Feb 2. Eur J Med Chem. 2021. PMID: 33582578
-
Tacrines as Therapeutic Agents for Alzheimer's Disease. V. Recent Developments.Chem Rec. 2021 Jan;21(1):162-174. doi: 10.1002/tcr.202000107. Epub 2020 Nov 10. Chem Rec. 2021. PMID: 33169934 Review.
Cited by
-
Acetylcholinesterase and monoamine oxidase-B inhibitory activities by ellagic acid derivatives isolated from Castanopsis cuspidata var. sieboldii.Sci Rep. 2021 Jul 6;11(1):13953. doi: 10.1038/s41598-021-93458-4. Sci Rep. 2021. PMID: 34230570 Free PMC article.
-
New Tetrahydroacridine Hybrids with Dichlorobenzoic Acid Moiety Demonstrating Multifunctional Potential for the Treatment of Alzheimer's Disease.Int J Mol Sci. 2020 May 26;21(11):3765. doi: 10.3390/ijms21113765. Int J Mol Sci. 2020. PMID: 32466601 Free PMC article.
-
New cyclopentaquinoline and 3,5-dichlorobenzoic acid hybrids with neuroprotection against oxidative stress for the treatment of Alzheimer's disease.J Enzyme Inhib Med Chem. 2023 Dec;38(1):2158822. doi: 10.1080/14756366.2022.2158822. J Enzyme Inhib Med Chem. 2023. PMID: 36629422 Free PMC article.
-
Novel Cyclopentaquinoline and Acridine Analogs as Multifunctional, Potent Drug Candidates in Alzheimer's Disease.Int J Mol Sci. 2022 May 24;23(11):5876. doi: 10.3390/ijms23115876. Int J Mol Sci. 2022. PMID: 35682556 Free PMC article.
-
Biological Evaluation, Molecular Docking, and SAR Studies of Novel 2-(2,4-Dihydroxyphenyl)-1H- Benzimidazole Analogues.Biomolecules. 2019 Dec 12;9(12):870. doi: 10.3390/biom9120870. Biomolecules. 2019. PMID: 31842463 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

