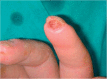
Classification, categorization and essential items for digital ulcer evaluation in systemic sclerosis: a DeSScipher/European Scleroderma Trials and Research group (EUSTAR) survey
- PMID: 30678703
- PMCID: PMC6346551
- DOI: 10.1186/s13075-019-1822-1
Classification, categorization and essential items for digital ulcer evaluation in systemic sclerosis: a DeSScipher/European Scleroderma Trials and Research group (EUSTAR) survey
Abstract
Background: A consensus on digital ulcer (DU) definition in systemic sclerosis (SSc) has been recently reached (Suliman et al., J Scleroderma Relat Disord 2:115-20, 2017), while for their evaluation, classification and categorisation, it is still missing. The aims of this study were to identify a set of essential items for digital ulcer (DU) evaluation, to assess if the existing DU classification was useful and feasible in clinical practice and to investigate if the new categorisation was preferred to the simple distinction of DU in recurrent and not recurrent, in patients with systemic sclerosis (SSc).
Methods: DeSScipher is the largest European multicentre study on SSc. It consists of five observational trials (OTs), and one of them, OT1, is focused on DU management. The DeSScipher OT1 items on DU that reached ≥ 60% of completion rate were administered to EUSTAR (European Scleroderma Trials and Research group) centres via online survey. Questions about feasibility and usefulness of the existing DU classification (DU due to digital pitting scars, to loss of tissue, derived from calcinosis and gangrene) and newly proposed categorisation (episodic, recurrent and chronic) were also asked.
Results: A total of 84/148 (56.8%) EUSTAR centres completed the questionnaire. DeSScipher items scored by ≥ 70% of the participants as essential and feasible for DU evaluation were the number of DU defined as a loss of tissue (level of agreement 92%), recurrent DU (84%) and number of new DU (74%). For 65% of the centres, the proposed classification of DU was considered useful and feasible in clinical practice. Moreover, 80% of the centres preferred the categorisation of DU in episodic, recurrent and chronic to simple distinction in recurrent/not recurrent DU.
Conclusions: For clinical practice, EUSTAR centres identified only three essential items for DU evaluation and considered the proposed classification and categorisation as useful and feasible. The set of items needs to be validated while further implementation of DU classification and categorisation is warranted.
Trial registration: Observational trial on DU (OT1) is one of the five trials of the DeSScipher project (ClinicalTrials.gov; OT1 Identifier: NCT01836263 , posted on April 19, 2013).
Keywords: Categorisation; Classification; Digital ulcers; Essential items; Systemic sclerosis.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval of DeSScipher OT1 has been obtained from all participating centres’ local ethics committees (project coordinator’s ethics board: Ethics Review board of the Justus-Liebig University Giessen, Germany, approval no 02/13; partners centres ethics review boards: University of Zurich, Switzerland; University of Paris, France; University of Florence Italy; The Second University of Naples, Italy; University of Basel, Switzerland; University College of London, United Kingdom; University of Berlin Charité, Germany; University of Pécs, Hungary; University of Leeds, United Kingdom; and contributors centres ethics boards (additional 21 centres)). Each patient signed a written informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors report personal fees and non-financial support by the European Community’s Framework Program 7 [FP7-HEALTH-2012.2.4.4-2 Observational trials in rare diseases]; grant agreement no. 305495.
OD: Consultancies, speaking fees, and honoraria < $10,000: AnaMar, Bayer, Boehringer Ingelheim, Catenion, CLS Behring, ChemomAb, Roche, GSK, Inventiva, Italfarmaco, Lilly, medac, Medscape, Mitsubishi Tanabe Pharma, MSD, Novartis, Pfizer, Sanofi, UCB Consultancies, speaking fees, and honoraria > $10,000: Bayer, Böhringer. Patents, licenses, or licensing fees: Patent mir-29 for the treatment of systemic sclerosis licensed
CPD: Consultancies, speaking fees, and honoraria < $10,000: Actelion, Bayer, Boehringer Ingelheim, Inventiva, Sanofi-Aventis, CSL Behring
SV: Consultancies, speaking fees, and honoraria < $10,000: Thermofisher, Abbvie, Boehringer-Ingelheim
YA: Consultancies, speaking fees, and honoraria < $10,000: Actelion, Boehringer, Roche, Sanofi, Inventiva, Medac, Bayer, BMS, Pfizer
MMC: Consultancies, speaking fees, and honoraria < $10,000: Actelion, BMS, Pfizer
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relat Disord. 2017;2:137–152. doi: 10.5301/jsrd.5000249. - DOI
-
- Matucci-Cerinic M, Krieg T, Guillevin L, Schwierin B, Rosenberg D, Cornelisse P, et al. Elucidating the burden of recurrent and chronic digital ulcers in systemic sclerosis: long-term results from the DUO Registry. Ann Rheum Dis. 2016;75:1770–1776. doi: 10.1136/annrheumdis-2015-208121. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous