Intravital imaging reveals systemic ezrin inhibition impedes cancer cell migration and lymph node metastasis in breast cancer
- PMID: 30678714
- PMCID: PMC6345049
- DOI: 10.1186/s13058-018-1079-7
Intravital imaging reveals systemic ezrin inhibition impedes cancer cell migration and lymph node metastasis in breast cancer
Abstract
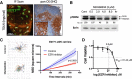
Background: Limited understanding of the cancer biology of metastatic sites is a major factor contributing to poor outcomes in cancer patients. The regional lymph nodes are the most common site of metastasis in most solid cancers and their involvement is a strong predictor of relapse in breast cancer (BC). We have previously shown that ezrin, a cytoskeletal-membrane linker protein, is associated with lymphovascular invasion and promotes metastatic progression in BC. However, the efficacy of pharmacological inhibition of ezrin in blocking cancer cell migration and metastasis remains unexplored in BC.
Methods: We quantified ezrin expression in a BC tissue microarray (n = 347) to assess its correlation with risk of relapse. Next, we developed a quantitative intravital microscopy (qIVM) approach, using a syngeneic lymphatic reporter mouse tumor model, to investigate the effect of systemic ezrin inhibition on cancer cell migration and metastasis.
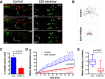
Results: We show that ezrin is expressed at significantly higher levels in lymph node metastases compared to matched primary tumors, and that a high tumor ezrin level is associated with increased risk of relapse in BC patients with regional disease. Using qIVM, we observe a subset of cancer cells that retain their invasive and migratory phenotype at the tumor-draining lymph node. We further show that systemic inhibition of ezrin, using a small molecule compound (NSC668394), impedes the migration of cancer cells in vivo. Furthermore, systemic ezrin inhibition leads to reductions in metastatic burden at the distal axillary lymph node and lungs.
Conclusions: Our findings demonstrate that the tumor ezrin level act as an independent biomarker in predicting relapse and provide a rationale for therapeutic targeting of ezrin to reduce the metastatic capacity of cancer cells in high-risk BC patients with elevated ezrin expression.
Keywords: Biomarker; Cell migration; Ezrin; Lymph node metastasis; Metastatic disease; Quantitative intravital imaging.
Conflict of interest statement
Ethics approval and consent to participate
Written informed consent was obtained from all patients and the studies were conducted in accordance with the Queen’s University Research Ethics Board consent guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Clucas J, Valderrama F. ERM proteins in cancer progression. J Cell Sci. 2014;127(Pt 2):267–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

