Increased Excitation-Inhibition Ratio Stabilizes Synapse and Circuit Excitability in Four Autism Mouse Models
- PMID: 30679017
- PMCID: PMC6733271
- DOI: 10.1016/j.neuron.2018.12.026
Increased Excitation-Inhibition Ratio Stabilizes Synapse and Circuit Excitability in Four Autism Mouse Models
Abstract
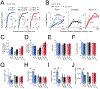
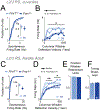
Distinct genetic forms of autism are hypothesized to share a common increase in excitation-inhibition (E-I) ratio in cerebral cortex, causing hyperexcitability and excess spiking. We provide a systematic test of this hypothesis across 4 mouse models (Fmr1-/y, Cntnap2-/-, 16p11.2del/+, Tsc2+/-), focusing on somatosensory cortex. All autism mutants showed reduced feedforward inhibition in layer 2/3 coupled with more modest, variable reduction in feedforward excitation, driving a common increase in E-I conductance ratio. Despite this, feedforward spiking, synaptic depolarization, and spontaneous spiking were largely normal. Modeling revealed that E and I conductance changes in each mutant were quantitatively matched to yield stable, not increased, synaptic depolarization for cells near spike threshold. Correspondingly, whisker-evoked spiking was not increased in vivo despite detectably reduced inhibition. Thus, elevated E-I ratio is a common circuit phenotype but appears to reflect homeostatic stabilization of synaptic drive rather than driving network hyperexcitability in autism.
Keywords: E-I ratio; Fragile X; autism; cerebral cortex; circuit excitability; excitation; homeostasis; inhibition; somatosensory cortex.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures




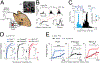
Comment in
-
Autism in the Balance: Elevated E-I Ratio as a Homeostatic Stabilization of Synaptic Drive.Neuron. 2019 Feb 20;101(4):543-545. doi: 10.1016/j.neuron.2019.01.033. Neuron. 2019. PMID: 30790531
-
The Neuroscientist Comments.Neuroscientist. 2019 Aug;25(4):285. doi: 10.1177/1073858419859506. Neuroscientist. 2019. PMID: 31514646 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

