Progressive cone and cone-rod dystrophies: clinical features, molecular genetics and prospects for therapy
- PMID: 30679166
- PMCID: PMC6709772
- DOI: 10.1136/bjophthalmol-2018-313278
Progressive cone and cone-rod dystrophies: clinical features, molecular genetics and prospects for therapy
Abstract
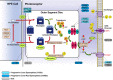
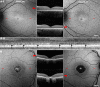
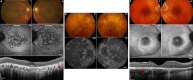
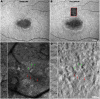
Progressive cone and cone-rod dystrophies are a clinically and genetically heterogeneous group of inherited retinal diseases characterised by cone photoreceptor degeneration, which may be followed by subsequent rod photoreceptor loss. These disorders typically present with progressive loss of central vision, colour vision disturbance and photophobia. Considerable progress has been made in elucidating the molecular genetics and genotype-phenotype correlations associated with these dystrophies, with mutations in at least 30 genes implicated in this group of disorders. We discuss the genetics, and clinical, psychophysical, electrophysiological and retinal imaging characteristics of cone and cone-rod dystrophies, focusing particularly on four of the most common disease-associated genes: GUCA1A, PRPH2, ABCA4 and RPGR Additionally, we briefly review the current management of these disorders and the prospects for novel therapies.
Keywords: dystrophy; genetics; imaging; retina.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
