FDG-PET and MRI in the Evolution of New-Onset Refractory Status Epilepticus
- PMID: 30679215
- PMCID: PMC7028610
- DOI: 10.3174/ajnr.A5929
FDG-PET and MRI in the Evolution of New-Onset Refractory Status Epilepticus
Abstract
Background and purpose: New-onset refractory status epilepticus is a clinical condition characterized by acute and prolonged pharmacoresistant seizures without a pre-existing relevant neurologic disorder, prior epilepsy, or clear structural, toxic, or metabolic cause. New-onset refractory status epilepticus is often associated with antineuronal antibodies and may respond to early immunosuppressive therapy, reflecting an inflammatory element of the condition. FDG-PET is a useful diagnostic tool in inflammatory and noninflammatory encephalitis. We report here FDG-PET findings in new-onset refractory status epilepticus and their correlation to disease activity, other imaging findings, and outcomes.
Materials and methods: Twelve patients who met the criteria for new-onset refractory status epilepticus and who had FDG-PET and MR imaging scans and electroencephalography at a single academic medical center between 2008 and 2017 were retrospectively identified. Images were independently reviewed by 2 radiologists specialized in nuclear imaging. Clinical characteristics and outcome measures were collected through chart review.
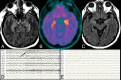
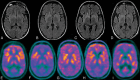
Results: Twelve patients underwent 21 FDG-PET scans and 50 MR imaging scans. Nine (75%) patients were positive for autoantibodies. All patients had identifiable abnormalities on the initial FDG-PET in the form of hypermetabolism (83%) and/or hypometabolism (42%). Eight (67%) had medial temporal involvement. All patients (n = 3) with N-methyl-D-aspartic acid receptor antibodies had profound bilateral occipital hypometabolism. Initial MR imaging findings were normal in 6 (50%) patients. Most patients had some degree of persistent hyper- (73%) or hypometabolism (45%) after immunosuppressive therapy. FDG-PET hypometabolism was predictive of poor outcome (mRS 4-6) at hospital discharge (P = .028).
Conclusions: Both FDG-PET hypometabolism and hypermetabolism are seen in the setting of new-onset refractory status epilepticus and may represent markers of disease activity.
© 2019 by American Journal of Neuroradiology.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
