Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study
- PMID: 30679233
- PMCID: PMC6344892
- DOI: 10.1136/bmj.l67
Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study
Abstract
Objective: To compare the effectiveness and safety of three non-tumour necrosis factor (TNF) α inhibitors (rituximab, abatacept, and tocilizumab) in the treatment of rheumatoid arthritis.
Design: Population based prospective study.
Setting: 53 university and 54 non-university clinical centres in France.
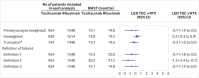
Participants: 3162 adults (>18 years) with rheumatoid arthritis according to 1987 American College of Rheumatology criteria, enrolled in one of the three French Society of Rheumatology registries; who had no severe cardiovascular disease, active or severe infections, or severe immunodeficiency, with follow-up of at least 24 months.
Intervention: Initiation of intravenous rituximab, abatacept, or tocilizumab for rheumatoid arthritis.
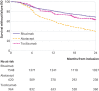
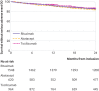
Main outcome measure: The primary outcome was drug retention without failure at 24 months. Failure was defined as all cause death; discontinuation of rituximab, abatacept, or tocilizumab; initiation of a new biologic or a combination of conventional disease modifying antirheumatic drugs; or increase in corticosteroid dose >10 mg/d compared with baseline at two successive visits. Because of non-proportional hazards, treatment effects are presented as life expectancy difference without failure (LEDwf), which measures the difference between average duration of survival without failure.
Results: Average durations of survival without failure were 19.8 months for rituximab, 15.6 months for abatacept, and 19.1 months for tocilizumab. Average durations were greater with rituximab (LEDwf 4.1, 95% confidence interval 3.1 to 5.2) and tocilizumab (3.5, 2.1 to 5.0) than with abatacept, and uncertainty about tocilizumab compared with rituximab was substantial (-0.7, -1.9 to 0.5). No evidence was found of difference between treatments for mean duration of survival without death, presence of cancer or serious infections, or major adverse cardiovascular events.
Conclusion: Among adults with refractory rheumatoid arthritis followed-up in routine practice, rituximab and tocilizumab were associated with greater improvements in outcomes at two years compared with abatacept.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: JEG reports personal fees from AbbVie, MSD, Janssen, Pfizer, UCB, and Lilly; grants and personal fees from Bristol-Meyers Squibb and Roche during the conduct of the study; and grants from Pfizer and Bristol-Meyers Squibb outside the submitted work. BC reports personal fees from Abbvie, Bristol-Meyers Squibb, Lilly, MSD, Janssen, Pfizer, Roche, Chigai, and Sanofi, during the conduct of the study, and grants from Abbvie, Bristol-Meyers Squibb, Lilly, MSD, Janssen, Pfizer, Roche, Chugai, and Sanofi outside the submitted work. MD reports grants and personal fees from Bristol-Meyers Squibb, AbbVie, Pfizer, Novartis, Lilly, UCB, Merck, and Janssen outside the submitted work. R-MF reports grants and personal fees from Roche, Chugai, Abbvie, and Pfizer, and personal fees from Bristol-Meyers Squibb outside the submitted work. AS reports grants, personal fees, and non-financial support from Roche, Chugai, and Bristol-Meyers Squibb outside the submitted work. TS reports and has been implicated in clinical trials for all the disease modifying antirheumatic drugs that are considered in the paper. JS reports personal fees from Roche, Chugai, and Bristol-Meyers Squibb during the conduct of the study, and personal fees from Roche, Chugai, Bristol-Meyers Squibb, UCB, GSK, LFB, Actelion, Pfizer, MSD, Novartis, Amgen, Hospira, AbbVie, Sandoz, Gilead, Lilly, Sanofi, Janssen, and Mylan outside the submitted work. OV reports personal fees from Bristol Myers Squibb, Roche, Chugai, MSD, Novartis, Pfizer, Abbvie, and Lilly outside the submitted work. AC reports personal fees from Bristol-Meyers Squibb, Chugai, Roche, Abbvie, MSD, Pfizer, and UCB outside the submitted work. XM received an honorarium for participation in boards without any relation to this study from Bristol-Meyers Squibb, GSK, Janssen, Pfizer, and UCB.
Figures
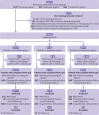


References
-
- Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ, British Society for Rheumatology Biologics Register Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum 2007;56:13-20. 10.1002/art.22331. - DOI - PubMed
-
- Cohen SB, Emery P, Greenwald MW, et al. REFLEX Trial Group Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006;54:2793-806. 10.1002/art.22025. - DOI - PubMed
-
- Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516-23. 10.1136/ard.2008.092932. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
