Exploring fast proton transfer events associated with lateral proton diffusion on the surface of membranes
- PMID: 30679274
- PMCID: PMC6377470
- DOI: 10.1073/pnas.1812351116
Exploring fast proton transfer events associated with lateral proton diffusion on the surface of membranes
Abstract
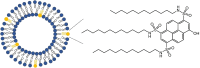
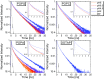
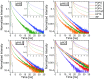
Proton diffusion (PD) across biological membranes is a fundamental process in many biological systems, and much experimental and theoretical effort has been employed for deciphering it. Here, we report on a spectroscopic probe, which can be tightly tethered to the membrane, for following fast (nanosecond) proton transfer events on the surface of membranes. Our probe is composed of a photoacid that serves as our light-induced proton source for the initiation of the PD process. We use our probe to follow PD, and its pH dependence, on the surface of lipid vesicles composed of a zwitterionic headgroup, a negative headgroup, a headgroup that is composed only from the negative phosphate group, or a positive headgroup without the phosphate group. We reveal that the PD kinetic parameters are highly sensitive to the nature of the lipid headgroup, ranging from a fast lateral diffusion at some membranes to the escape of protons from surface to bulk (and vice versa) at others. By referring to existing theoretical models for membrane PD, we found that while some of our results confirm the quasi-equilibrium model, other results are in line with the nonequilibrium model.
Keywords: excited-state proton transfer; lipid vesicles; molecular dynamics; photoacid; proton diffusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
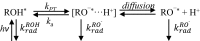

References
-
- Mulkidjanian AY, Heberle J, Cherepanov DA. Protons @ interfaces: Implications for biological energy conversion. Biochim Biophys Acta. 2006;1757:913–930. - PubMed
-
- Williams RJP. Proton circuits in biological energy interconversions. Annu Rev Biophys Biophys Chem. 1988;17:71–97. - PubMed
-
- Agmon N. The Grotthuss mechanism. Chem Phys Lett. 1995;244:456–462.
-
- Knight C, Voth GA. The curious case of the hydrated proton. Acc Chem Res. 2012;45:101–109. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

