A Randomized Controlled Trial on the Safety and Efficacy of Exenatide Therapy for the Inpatient Management of General Medicine and Surgery Patients With Type 2 Diabetes
- PMID: 30679302
- PMCID: PMC6905476
- DOI: 10.2337/dc18-1760
A Randomized Controlled Trial on the Safety and Efficacy of Exenatide Therapy for the Inpatient Management of General Medicine and Surgery Patients With Type 2 Diabetes
Abstract
Objective: This multicenter, open-label, randomized trial examined the safety and efficacy of exenatide alone or in combination with basal insulin in non-critically ill patients with type 2 diabetes (T2D).
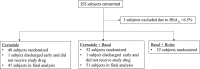
Research design and methods: A total of 150 patients with blood glucose (BG) between 140 and 400 mg/dL, treated at home with diet, oral agents, or insulin at a total daily dose <0.5 units/kg, were randomized to exenatide alone (5 μg twice daily), exenatide plus basal insulin, or a basal-bolus insulin regimen. The primary end point was difference in mean daily BG concentration among groups.
Results: Mean daily BG was similar between patients treated with exenatide plus basal and a basal-bolus regimen (154 ± 39 vs. 166 ± 40 mg/dL, P = 0.31), and exenatide plus basal resulted in lower daily BG than did exenatide alone (177 ± 41 mg/dL, P = 0.02). Exenatide plus basal resulted in a higher proportion of BG levels in target range between 70 and 180 mg/dL compared with exenatide and basal-bolus (78% vs. 62% vs. 63%, respectively, P = 0.023). More patients in the exenatide and exenatide plus basal groups experienced nausea or vomiting than in the basal-bolus group (10% vs. 11% vs. 2%, P = 0.17), with three patients (6%) discontinued exenatide owing to adverse events. There were no differences in hypoglycemia <54 mg/dL (2% vs. 0% vs. 4%, P = 0.77) or length of stay (5 vs. 4 vs. 4 days, P = 0.23) among basal plus exenatide, exenatide, and basal-bolus groups.
Conclusions: The results of this pilot study indicate that exenatide alone or in combination with basal insulin is safe and effective for the management of hospitalized general medical and surgical patients with T2D.
Trial registration: ClinicalTrials.gov NCT02455076.
© 2019 by the American Diabetes Association.
Figures

References
-
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 - PubMed
-
- Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA 2003;290:2041–2047 - PubMed
-
- Van den Berghe G, Wouters PJ, Bouillon R, et al. . Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med 2003;31:359–366 - PubMed
-
- Pomposelli JJ, Baxter JK III, Babineau TJ, et al. . Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr 1998;22:77–81 - PubMed
-
- Kosiborod M, Inzucchi SE, Krumholz HM, et al. . Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med 2009;169:438–446 - PubMed

