Universal fluorescent sensors of high-affinity iron transport, applied to ESKAPE pathogens
- PMID: 30679312
- PMCID: PMC6433069
- DOI: 10.1074/jbc.RA118.006921
Universal fluorescent sensors of high-affinity iron transport, applied to ESKAPE pathogens
Abstract
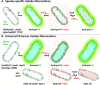
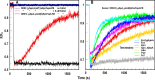
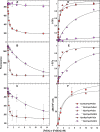
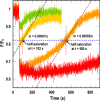
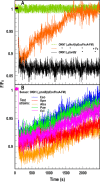
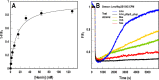
Sensitive assays of biochemical specificity, affinity, and capacity are valuable both for basic research and drug discovery. We created fluorescent sensors that monitor high-affinity binding reactions and used them to study iron acquisition by ESKAPE bacteria, which are frequently responsible for antibiotic-resistant infections. By introducing site-directed Cys residues in bacterial iron transporters and modifying them with maleimide fluorophores, we generated living cells or purified proteins that bind but do not transport target compounds. These constructs sensitively detected ligand concentrations in solution, enabling accurate, real-time spectroscopic analysis of membrane transport by other cells. We assessed the efficacy of these "fluorescent decoy" (FD) sensors by characterizing active iron transport in the ESKAPE bacteria. The FD sensors monitored uptake of both ferric siderophores and hemin by the pathogens. An FD sensor for a particular ligand was universally effective in observing the uptake of that compound by all organisms we tested. We adapted the FD sensors to microtiter format, where they allow high-throughput screens for chemicals that block iron uptake, without genetic manipulations of the virulent target organisms. Hence, screening assays with FD sensors facilitate studies of mechanistic biochemistry, as well as discovery of chemicals that inhibit prokaryotic membrane transport. With appropriate design, FD sensors are potentially applicable to any pro- or eukaryotic high-affinity ligand transport process.
Keywords: ESKAPE pathogen; fluorescence; heme; iron; ligand-binding protein; membrane transport; pathogenesis; siderophore; spectroscopy.
© 2019 Chakravorty et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Tacconelli E., and Magrini N. (2017) Global Priority List of Antibiotic-resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics, World Health Organization, Geneva
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

