Everolimus plus Exemestane for Hormone Receptor-Positive Advanced Breast Cancer: A PAM50 Intrinsic Subtype Analysis of BOLERO-2
- PMID: 30679318
- PMCID: PMC6656445
- DOI: 10.1634/theoncologist.2018-0407
Everolimus plus Exemestane for Hormone Receptor-Positive Advanced Breast Cancer: A PAM50 Intrinsic Subtype Analysis of BOLERO-2
Abstract
Background: The prognostic and predictive value of the two nonluminal (human epidermal growth factor receptor 2 [HER2]-enriched and basal-like) subtypes within advanced hormone receptor-positive (HR+) breast cancer is currently unknown.
Materials and methods: This study retrospectively analyzed 261 tumors (80.7% primary; 19.3% metastatic) from the BOLERO-2 study; BOLERO-2 randomized 724 patients with advanced HR+/HER2-negative breast cancer to everolimus plus exemestane or placebo plus exemestane. Tumors were classified using a PAM50 subtype predictor. Multivariable Cox regression analyses tested the independent prognostic significance of PAM50, and associations between PAM50 subtypes and treatment upon progression-free survival (PFS) were evaluated.
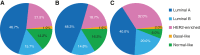
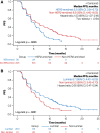
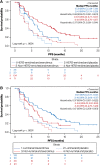
Results: Subtype distribution was 46.7% luminal A (n = 122), 21.5% HER2-enriched (n = 56), 15.7% luminal B (n = 41), 14.2% normal-like (n = 37), and 1.9% basal-like (n = 5); HER2-enriched subtypes were more common in metastatic versus primary tumors (32.0% vs. 18.7%; p = .038). Median PFS differences between luminal and nonluminal (6.7 vs. 5.2 months; adjusted hazard ratio, 0.66; 95% confidence interval [CI], 0.47-0.94; p = .020) and HER2-enriched and non-HER2-enriched subtypes (5.2 vs. 6.2 months; adjusted hazard ratio, 1.53; 95% CI, 1.07-2.19; p = .019) were significant. Everolimus plus exemestane significantly improved median PFS versus placebo plus exemestane among patients with HER2-enriched tumors (5.8 vs. 4.1 months; adjusted hazard ratio, 0.49; 95% CI, 0.26-0.90; p = .034); however, the association between HER2-enriched tumors and everolimus benefit was nonsignificant (p = .433).
Conclusion: The HER2-enriched subtype was identified in a substantial proportion of advanced HR+/HER2-negative breast tumors, and was a consistent biomarker of poor prognosis. Tailored therapies are therefore needed for HER2-enriched tumors in the advanced HR+/HER2-negative breast cancer setting.
Implications for practice: Using 261 tumor samples from the BOLERO-2 phase III clinical trial, this study shows that a substantial proportion (20%-30%) of hormone receptor-positive (HR+)/human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancers do not have a luminal A or B gene expression profile. This group of patients with nonluminal disease has a poor survival outcome regardless of the addition of everolimus to exemestane. This is the second study that confirms the prognostic value of this biomarker. Overall, these findings indicate a necessity to design novel clinical trials targeting nonluminal disease within HR+/HER2-negative breast cancer.
摘要
背景。目前尚不清楚激素受体阳性 (HR+) 的晚期乳腺癌中两种非管腔 [人表皮生长因子受体 2 (HER2) ‐ 过表达和基底细胞样]亚型的预后和预测价值。
材料和方法。本研究回顾性分析了 BOLERO‐2 研究中的 261 例肿瘤(80.7% 原发;19.3% 转移);BOLERO‐2 将 724 例晚期 HR +/HER2 阴性的晚期乳腺癌患者随机分配至依维莫司加依西美坦或安慰剂加依西美坦。使用 PAM50 亚型预测因子对肿瘤进行分类。多变量 Cox 回归分析检验了 PAM50 的独立预后意义,并评估了 PAM50 亚型与治疗无进展生存期 (PFS) 之间的关联。
结果。亚型分布为 46.7% 管腔 A 型(n = 122),21.5% HER2 过表达(n = 56),15.7% 管腔 B 型(n = 41),14.2% 正常样型(n = 37),以及 1.9% 基底细胞样型(n = 5);HER2 过表达的亚型在转移性肿瘤中较原发性肿瘤更常见(32.0% vs. 18.7%;p = 0.038)。中位 PFS方面,管腔型和非管腔型之间[6.7 vs. 5.2 个月;校正风险比,0.66;95% 置信区间 (CI),0.47‐0.94;p = 0.020]以及 HER2 过表达和非 HER2 过表达亚型之间(5.2 vs. 6.2个月;校正风险比,1.53;95%CI,1.07‐2.19;p = 0.019)的差异明显。在 HER2 过表达的肿瘤患者中,依维莫司加依西美坦与安慰剂加依西美坦相比显著改善了中位 PFS(5.8 vs. 4.1 个月;校正风险比,0.49;95%CI,0.26‐0.90;p = 0.034);然而,HER2 过表达肿瘤与依维莫司治疗受益之间的关联并不显著(p = 0.433)。
结论。 HER2 过表达亚型在晚期 HR +/HER2 阴性乳腺肿瘤中占有相当大比例,并且是预后不良的一致性生物标志物。因此,对于晚期 HR +/HER2 阴性乳腺癌患者中 HER2 过表达的肿瘤需要个体化定制疗法。
实践意义:使用来自 BOLERO‐2 III 期临床试验的 261 份肿瘤样本,本研究显示相当大部分(20% ‐ 30%)激素受体阳性 (HR+)/人表皮生长因子受体 2 (HER2)‐阴性的晚期乳腺癌不具有管腔 A 型或 B 型基因表达谱。无论在依西美坦治疗中是否加入依维莫司,这组患有非管腔型肿瘤的患者的生存结果都很差。这是第二项证实了该生物标志物的预后价值的研究。总体而言,这些研究结果表明有必要设计针对 HR +/HER2 阴性非管腔型乳腺癌的新型临床试验。
Keywords: Advanced breast cancer; Everolimus; Exemestane; Intrinsic subtype; Mammalian target of rapamycin.
© AlphaMed Press 2019.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Similar articles
-
A Prognostic Model Based on PAM50 and Clinical Variables (PAM50MET) for Metastatic Hormone Receptor-positive HER2-negative Breast Cancer.Clin Cancer Res. 2020 Dec 1;26(23):6141-6148. doi: 10.1158/1078-0432.CCR-20-2793. Epub 2020 Sep 22. Clin Cancer Res. 2020. PMID: 32962980 Clinical Trial.
-
Efficacy and safety of everolimus plus exemestane in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer: Results of the single-arm, phase IIIB 4EVER trial.Int J Cancer. 2019 Feb 15;144(4):877-885. doi: 10.1002/ijc.31738. Epub 2018 Oct 30. Int J Cancer. 2019. PMID: 29992557 Free PMC article. Clinical Trial.
-
Everolimus Plus Exemestane vs Everolimus or Capecitabine Monotherapy for Estrogen Receptor-Positive, HER2-Negative Advanced Breast Cancer: The BOLERO-6 Randomized Clinical Trial.JAMA Oncol. 2018 Oct 1;4(10):1367-1374. doi: 10.1001/jamaoncol.2018.2262. JAMA Oncol. 2018. PMID: 29862411 Free PMC article. Clinical Trial.
-
Everolimus-based combination therapies for HR+, HER2- metastatic breast cancer.Cancer Treat Rev. 2018 Sep;69:204-214. doi: 10.1016/j.ctrv.2018.07.013. Epub 2018 Jul 23. Cancer Treat Rev. 2018. PMID: 30092555 Review.
-
Everolimus versus alpelisib in advanced hormone receptor-positive HER2-negative breast cancer: targeting different nodes of the PI3K/AKT/mTORC1 pathway with different clinical implications.Breast Cancer Res. 2020 Apr 6;22(1):33. doi: 10.1186/s13058-020-01271-0. Breast Cancer Res. 2020. PMID: 32252811 Free PMC article. Review.
Cited by
-
Early Changes of the Standardized Uptake Values (SUVmax) Predict the Efficacy of Everolimus-Exemestane in Patients with Hormone Receptor-Positive Metastatic Breast Cancer.Cancers (Basel). 2020 Nov 10;12(11):3314. doi: 10.3390/cancers12113314. Cancers (Basel). 2020. PMID: 33182575 Free PMC article.
-
Clinical Implications of Breast Cancer Intrinsic Subtypes.Adv Exp Med Biol. 2025;1464:435-448. doi: 10.1007/978-3-031-70875-6_21. Adv Exp Med Biol. 2025. PMID: 39821037 Review.
-
Role of PI3K/Akt/mTOR pathway in mediating endocrine resistance: concept to clinic.Explor Target Antitumor Ther. 2022;3(2):172-199. doi: 10.37349/etat.2022.00078. Epub 2022 Apr 24. Explor Target Antitumor Ther. 2022. PMID: 36046843 Free PMC article. Review.
-
Role of Intrinsic Subtype Analysis with PAM50 in Hormone Receptors Positive HER2 Negative Metastatic Breast Cancer: A Systematic Review.Int J Mol Sci. 2022 Jun 25;23(13):7079. doi: 10.3390/ijms23137079. Int J Mol Sci. 2022. PMID: 35806079 Free PMC article.
-
Correlative Biomarker Analysis of Intrinsic Subtypes and Efficacy Across the MONALEESA Phase III Studies.J Clin Oncol. 2021 May 1;39(13):1458-1467. doi: 10.1200/JCO.20.02977. Epub 2021 Mar 26. J Clin Oncol. 2021. PMID: 33769862 Free PMC article. Clinical Trial.
References
-
- Cardoso F, Costa A, Senkus E et al. 3rd ESO‐ESMO international consensus guidelines for advanced breast cancer (ABC 3). Breast 2017;31:244–259. - PubMed
-
- Rugo HS, Rumble BR, Macrae E et al. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 2016;34:3069–3103. - PubMed
-
- Prat A, Pineda E, Adamo B et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015;24(suppl 2):S26–S35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

