Spatio-temporal overview of neuroinflammation in an experimental mouse stroke model
- PMID: 30679481
- PMCID: PMC6345915
- DOI: 10.1038/s41598-018-36598-4
Spatio-temporal overview of neuroinflammation in an experimental mouse stroke model
Abstract
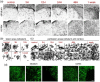
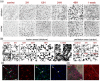
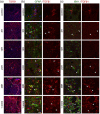
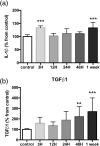
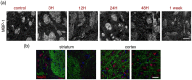
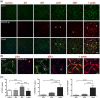
After ischemic stroke, in the lesion core as well as in the ischemic penumbra, evolution of tissue damage and repair is strongly affected by neuroinflammatory events that involve activation of local specialized glial cells, release of inflammatory mediators, recruiting of systemic cells and vascular remodelling. To take advantage of this intricate response in the quest to devise new protective therapeutic strategies we need a better understanding of the territorial and temporal interplay between stroke-triggered inflammatory and cell death-inducing processes in both parenchymal and vascular brain cells. Our goal is to describe structural rearrangements and functional modifications occurring in glial and vascular cells early after an acute ischemic stroke. Low and high scale mapping of the glial activation on brain sections of mice subjected to 30 minutes middle cerebral artery occlusion (MCAO) was correlated with that of the neuronal cell death, with markers for microvascular changes and with markers for pro-inflammatory (IL-1β) and reparative (TGFβ1) cytokines. Our results illustrate a time-course of the neuroinflammatory response starting at early time-points (1 h) and up to one week after MCAO injury in mice, with an accurate spatial distribution of the observed phenomena.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

