Tissue acidosis does not mediate the hypoxia selectivity of [64Cu][Cu(ATSM)] in the isolated perfused rat heart
- PMID: 30679497
- PMCID: PMC6346098
- DOI: 10.1038/s41598-018-36145-1
Tissue acidosis does not mediate the hypoxia selectivity of [64Cu][Cu(ATSM)] in the isolated perfused rat heart
Abstract
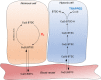
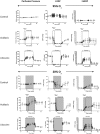
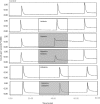
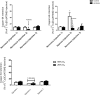
Copper-64-Diacetyl-bis(N4-methylthiosemicarbazone) [64Cu][Cu(ATSM)] is a hypoxia-targeting PET tracer with applications in oncology and cardiology. Upon entering a hypoxic cell, [64Cu][Cu(II)(ATSM)] is reduced to a putative [64Cu][Cu(I)(ATSM)]- species which dissociates to deposit radiocopper, thereby providing hypoxic contrast. This process may be dependent upon protonation arising from intracellular acidosis. Since acidosis is a hallmark of ischemic tissue and tumors, the hypoxia specificity of [64Cu][Cu(ATSM)] may be confounded by changes in intracellular pH. We have therefore determined the influence of intracellular pH on [64Cu][Cu(ATSM)] pharmacokinetics. Using isolated perfused rat hearts, acidosis was induced using an ammonium pre-pulse method, with and without hypoxic buffer perfusion. Cardiac [64Cu][Cu(ATSM)] pharmacokinetics were determined using NaI detectors, with intracellular pH and cardiac energetics monitored in parallel by 31P NMR. To distinguish direct acidotic effects on tracer pharmacokinetics from acidosis-induced hypocontractility, parallel studies used lidocaine perfusion to abolish cardiac contraction. Hypoxic myocardium trapped [64Cu][Cu(ATSM)] despite no evidence of it being acidotic when characterised by 31P NMR. Independent induction of tissue acidosis had no direct effect on [64Cu][Cu(ATSM)] pharmacokinetics in either normoxic or hypoxic hearts, beyond decreasing cardiac oxygen consumption to alleviate hypoxia and decrease tracer retention, leading us to conclude that tissue acidosis does not mediate the hypoxia selectivity of [64Cu][Cu(ATSM)].
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Delineation of hypoxia in canine myocardium using PET and copper(II)-diacetyl-bis(N(4)-methylthiosemicarbazone).J Nucl Med. 2002 Nov;43(11):1557-69. J Nucl Med. 2002. PMID: 12411560
-
Cardiac hypoxia imaging: second-generation analogues of 64Cu-ATSM.J Nucl Med. 2014 Mar;55(3):488-94. doi: 10.2967/jnumed.113.129015. Epub 2014 Jan 13. J Nucl Med. 2014. PMID: 24421288 Free PMC article.
-
Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential.J Nucl Med. 1997 Jul;38(7):1155-60. J Nucl Med. 1997. PMID: 9225812
-
Hypoxia imaging and theranostic potential of [64Cu][Cu(ATSM)] and ionic Cu(II) salts: a review of current evidence and discussion of the retention mechanisms.EJNMMI Res. 2020 Apr 9;10(1):33. doi: 10.1186/s13550-020-00621-5. EJNMMI Res. 2020. PMID: 32274601 Free PMC article. Review.
-
Copper(II) diacetyl-di(N4-methylthiosemicarbazone).2004 Nov 10 [updated 2005 Jul 31]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2004 Nov 10 [updated 2005 Jul 31]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641533 Free Books & Documents. Review.
Cited by
-
Elevated Na is a dynamic and reversible modulator of mitochondrial metabolism in the heart.Nat Commun. 2024 May 20;15(1):4277. doi: 10.1038/s41467-024-48474-z. Nat Commun. 2024. PMID: 38769288 Free PMC article.
-
Detecting Validated Intracellular ROS Generation with 18F-dihydroethidine-Based PET.Mol Imaging Biol. 2022 Jun;24(3):377-383. doi: 10.1007/s11307-021-01683-0. Epub 2021 Nov 24. Mol Imaging Biol. 2022. PMID: 34820762 Free PMC article.
-
Novel Tracers and Radionuclides in PET Imaging.Radiol Clin North Am. 2021 Sep;59(5):887-918. doi: 10.1016/j.rcl.2021.05.012. Radiol Clin North Am. 2021. PMID: 34392925 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

