Drug repositioning for dengue haemorrhagic fever by integrating multiple omics analyses
- PMID: 30679503
- PMCID: PMC6346040
- DOI: 10.1038/s41598-018-36636-1
Drug repositioning for dengue haemorrhagic fever by integrating multiple omics analyses
Abstract
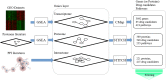
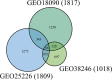
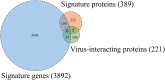
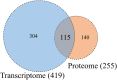
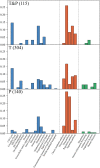
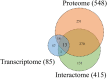
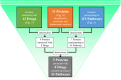
To detect drug candidates for dengue haemorrhagic fever (DHF), we employed a computational drug repositioning method to perform an integrated multiple omics analysis based on transcriptomic, proteomic, and interactomic data. We identified 3,892 significant genes, 389 proteins, and 221 human proteins by transcriptomic analysis, proteomic analysis, and human-dengue virus protein-protein interactions, respectively. The drug candidates were selected using gene expression profiles for inverse drug-disease relationships compared with DHF patients and healthy controls as well as interactomic relationships between the signature proteins and chemical compounds. Integrating the results of the multiple omics analysis, we identified eight candidates for drug repositioning to treat DHF that targeted five proteins (ACTG1, CALR, ERC1, HSPA5, SYNE2) involved in human-dengue virus protein-protein interactions, and the signature proteins in the proteomic analysis mapped to significant pathways. Interestingly, five of these drug candidates, valparoic acid, sirolimus, resveratrol, vorinostat, and Y-27632, have been reported previously as effective treatments for flavivirus-induced diseases. The computational approach using multiple omics data for drug repositioning described in this study can be used effectively to identify novel drug candidates.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

