Open-Source Multiparametric Optocardiography
- PMID: 30679527
- PMCID: PMC6346041
- DOI: 10.1038/s41598-018-36809-y
Open-Source Multiparametric Optocardiography
Abstract
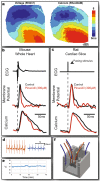
Since the 1970s fluorescence imaging has become a leading tool in the discovery of mechanisms of cardiac function and arrhythmias. Gradual improvements in fluorescent probes and multi-camera technology have increased the power of optical mapping and made a major impact on the field of cardiac electrophysiology. Tandem-lens optical mapping systems facilitated simultaneous recording of multiple parameters characterizing cardiac function. However, high cost and technological complexity restricted its proliferation to the wider biological community. We present here, an open-source solution for multiple-camera tandem-lens optical systems for multiparametric mapping of transmembrane potential, intracellular calcium dynamics and other parameters in intact mouse hearts and in rat heart slices. This 3D-printable hardware and Matlab-based RHYTHM 1.2 analysis software are distributed under an MIT open-source license. Rapid prototyping permits the development of inexpensive, customized systems with broad functionality, allowing wider application of this technology outside biomedical engineering laboratories.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

