5-Fluorouracil Induces Enteric Neuron Death and Glial Activation During Intestinal Mucositis via a S100B-RAGE-NFκB-Dependent Pathway
- PMID: 30679569
- PMCID: PMC6345953
- DOI: 10.1038/s41598-018-36878-z
5-Fluorouracil Induces Enteric Neuron Death and Glial Activation During Intestinal Mucositis via a S100B-RAGE-NFκB-Dependent Pathway
Abstract
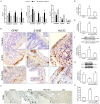
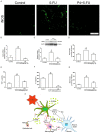
5-Fluorouracil (5-FU) is an anticancer agent whose main side effects include intestinal mucositis associated with intestinal motility alterations maybe due to an effect on the enteric nervous system (ENS), but the underlying mechanism remains unclear. In this report, we used an animal model to investigate the participation of the S100B/RAGE/NFκB pathway in intestinal mucositis and enteric neurotoxicity caused by 5-FU (450 mg/kg, IP, single dose). 5-FU induced intestinal damage observed by shortened villi, loss of crypt architecture and intense inflammatory cell infiltrate as well as increased GFAP and S100B co-expression and decreased HuC/D protein expression in the small intestine. Furthermore, 5-FU increased RAGE and NFκB NLS immunostaining in enteric neurons, associated with a significant increase in the nitrite/nitrate, IL-6 and TNF-α levels, iNOS expression and MDA accumulation in the small intestine. We provide evidence that 5-FU induces reactive gliosis and reduction of enteric neurons in a S100B/RAGE/NFκB-dependent manner, since pentamidine, a S100B inhibitor, prevented 5-FU-induced neuronal loss, enteric glia activation, intestinal inflammation, oxidative stress and histological injury.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

