Curcumin inhibits the TGF-β1-dependent differentiation of lung fibroblasts via PPARγ-driven upregulation of cathepsins B and L
- PMID: 30679571
- PMCID: PMC6345753
- DOI: 10.1038/s41598-018-36858-3
Curcumin inhibits the TGF-β1-dependent differentiation of lung fibroblasts via PPARγ-driven upregulation of cathepsins B and L
Abstract
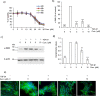
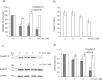
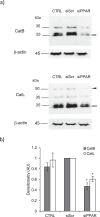
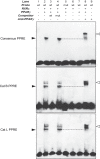
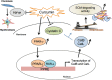
Pulmonary fibrosis is a progressive disease characterized by a widespread accumulation of myofibroblasts and extracellular matrix components. Growing evidences support that cysteine cathepsins, embracing cathepsin B (CatB) that affects TGF-β1-driven Smad pathway, along with their extracellular inhibitor cystatin C, participate in myofibrogenesis. Here we established that curcumin, a potent antifibrotic drug used in traditional Asian medicine, impaired the expression of both α-smooth muscle actin and mature TGF-β1 and inhibited the differentiation of human lung fibroblasts (CCD-19Lu cells). Curcumin induced a compelling upregulation of CatB and CatL. Conversely cystatin C was downregulated, which allowed the recovery of the peptidase activity of secreted cathepsins and the restoration of the proteolytic balance. Consistently, the amount of both insoluble and soluble type I collagen decreased, reaching levels similar to those observed for undifferentiated fibroblasts. The signaling pathways activated by curcumin were further examined. Curcumin triggered the expression of nuclear peroxisome proliferator-activated receptor γ (PPARγ). Contrariwise PPARγ inhibition, either by an antagonist (2-chloro-5-nitro-N-4-pyridinyl-benzamide) or by RNA silencing, restored TGF-β1-driven differentiation of curcumin-treated CCD-19Lu cells. PPARγ response element (PPRE)-like sequences were identified in the promoter regions of both CatB and CatL. Finally, we established that the transcriptional induction of CatB and CatL depends on the binding of PPARγ to PPRE sequences as a PPARγ/Retinoid X Receptor-α heterodimer.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

