Fibrogenic Gene Expression in Hepatic Stellate Cells Induced by HCV and HIV Replication in a Three Cell Co-Culture Model System
- PMID: 30679661
- PMCID: PMC6345841
- DOI: 10.1038/s41598-018-37071-y
Fibrogenic Gene Expression in Hepatic Stellate Cells Induced by HCV and HIV Replication in a Three Cell Co-Culture Model System
Abstract
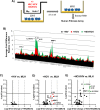
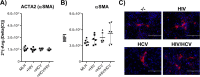
Retrospective studies indicate that co-infection of hepatitis C virus (HCV) and human immunodeficiency virus (HIV) accelerates hepatic fibrosis progression. We have developed a co-culture system (MLH) comprising primary macrophages, hepatic stellate cells (HSC, LX-2), and hepatocytes (Huh-7), permissive for active replication of HCV and HIV, and assessed the effect of these viral infections on the phenotypic changes and fibrogenic gene expression in LX-2 cells. We detected distinct morphological changes in LX-2 cells within 24 hr post-infection with HCV, HIV or HCV/HIV in MLH co-cultures, with migration enhancement phenotypes. Human fibrosis microarrays conducted using LX-2 cell RNA derived from MLH co-culture conditions, with or without HCV and HIV infection, revealed novel insights regarding the roles of these viral infections on fibrogenic gene expression in LX-2 cells. We found that HIV mono-infection in MLH co-culture had no impact on fibrogenic gene expression in LX-2 cells. HCV infection of MLH co-culture resulted in upregulation (>1.9x) of five fibrogenic genes including CCL2, IL1A, IL1B, IL13RA2 and MMP1. These genes were upregulated by HCV/HIV co-infection but in a greater magnitude. Conclusion: Our results indicate that HIV-infected macrophages accelerate hepatic fibrosis during HCV/HIV co-infection by amplifying the expression of HCV-dependent fibrogenic genes in HSC.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

