Electronic and optical properties of lead-free hybrid double perovskites for photovoltaic and optoelectronic applications
- PMID: 30679678
- PMCID: PMC6345881
- DOI: 10.1038/s41598-018-37132-2
Electronic and optical properties of lead-free hybrid double perovskites for photovoltaic and optoelectronic applications
Abstract
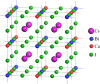
Developing of lead-free double perovskites have drawn significant interest for photovoltaics and optoelectronics as the materials have the potential to avoid toxicity and instability issues associated with lead-based organometallic perovskites. In this study, we report the optoelectronic properties of a new group of non-toxic lead-free organic-inorganic halide double perovskites composed of caesium (Cs), methylammonium (MA) or formamidinium (FA) with bismuth (Bi) and metal copper (Cu). We perform density functional theory investigations to calculate the structural, electronic and optical properties of 18 Pb-free compounds, ABiCuX6 [A = Cs2, (MA)2, (FA)2, CsMA, CsFA, MAFA; X = I, Br, Cl] to predict their suitability in photovoltaic and optoelectronic applications. We found that the considered compounds are semiconductors with a tunable band gap characteristics that are suitable for some devices like light emitting diodes. In addition to this, the high dielectric constant, high absorption, high optical conductivity and low reflectivity suggest that the materials have the potential in a wide range of optoelectronic applications including solar cells. Furthermore, we predict that the organic-inorganic hybrid double perovskite (FA)2BiCuI6 is the best candidate in photovoltaic and optoelectronic applications as the material has superior optical and electronic properties.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

