Sonic hedgehog signaling is negatively regulated in reactive astrocytes after forebrain stab injury
- PMID: 30679745
- PMCID: PMC6345977
- DOI: 10.1038/s41598-018-37555-x
Sonic hedgehog signaling is negatively regulated in reactive astrocytes after forebrain stab injury
Abstract
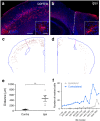
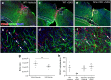
Following injury to the central nervous system, astrocytes perform critical and complex functions that both promote and antagonize neural repair. Understanding the molecular signaling pathways that coordinate their diverse functional properties is key to developing effective therapeutic strategies. In the healthy, adult CNS, Sonic hedgehog (Shh) signaling is active in mature, differentiated astrocytes. Shh has been shown to undergo injury-induced upregulation and promote neural repair. Here, we investigated whether Shh signaling mediates astrocyte response to injury. Surprisingly, we found that following an acute, focal injury, reactive astrocytes exhibit a pronounced reduction in Shh activity in a spatiotemporally-defined manner. Shh signaling is lost in reactive astrocytes at the lesion site, but persists in mild to moderately reactive astrocytes in distal tissues. Nevertheless, local pharmacological activation of the Shh pathway in astrocytes mitigates inflammation, consistent with a neuroprotective role for Shh signaling after injury. Interestingly, we find that Shh signaling is restored to baseline levels two weeks after injury, a time during which acute inflammation has largely subsided and lesions have matured. Taken together, these data suggest that endogenous Shh signaling in astrocytes is dynamically regulated in a context dependent manner. In addition, exogenous activation of the Shh pathway promotes neuroprotection mediated by reactive astrocytes.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

