Optimization of HER3 expression imaging using affibody molecules: Influence of chelator for labeling with indium-111
- PMID: 30679757
- PMCID: PMC6345776
- DOI: 10.1038/s41598-018-36827-w
Optimization of HER3 expression imaging using affibody molecules: Influence of chelator for labeling with indium-111
Abstract
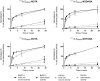
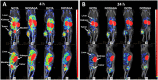
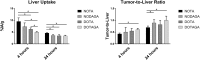
Radionuclide molecular imaging of human epidermal growth factor receptor 3 (HER3) expression using affibody molecules could be used for patient stratification for HER3-targeted cancer therapeutics. We hypothesized that the properties of HER3-targeting affibody molecules might be improved through modification of the radiometal-chelator complex. Macrocyclic chelators NOTA (1,4,7-triazacyclononane-N,N',N''-triacetic acid), NODAGA (1-(1,3-carboxypropyl)-4,7-carboxymethyl-1,4,7-triazacyclononane), DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid), and DOTAGA (1,4,7,10-tetraazacyclododececane,1-(glutaric acid)-4,7,10-triacetic acid) were conjugated to the C-terminus of anti-HER3 affibody molecule Z08698 and conjugates were labeled with indium-111. All conjugates bound specifically and with picomolar affinity to HER3 in vitro. In mice bearing HER3-expressing xenografts, no significant difference in tumor uptake between the conjugates was observed. Presence of the negatively charged 111In-DOTAGA-complex resulted in the lowest hepatic uptake and the highest tumor-to-liver ratio. In conclusion, the choice of chelator influences the biodistribution of indium-111 labeled anti-HER3 affibody molecules. Hepatic uptake of anti-HER3 affibody molecules could be reduced by the increase of negative charge of the radiometal-chelator complex on the C-terminus without significantly influencing the tumor uptake.
Conflict of interest statement
S.S., V.T. and A.O. own stock in Affibody A.B. S.S.R., C.D.L., M.R., T.Z.B., A.K.G., J.L. declare no potential conflict of interest.
Figures


Similar articles
-
Molecular Design of HER3-Targeting Affibody Molecules: Influence of Chelator and Presence of HEHEHE-Tag on Biodistribution of 68Ga-Labeled Tracers.Int J Mol Sci. 2019 Mar 2;20(5):1080. doi: 10.3390/ijms20051080. Int J Mol Sci. 2019. PMID: 30832342 Free PMC article.
-
Increase in negative charge of 68Ga/chelator complex reduces unspecific hepatic uptake but does not improve imaging properties of HER3-targeting affibody molecules.Sci Rep. 2019 Nov 27;9(1):17710. doi: 10.1038/s41598-019-54149-3. Sci Rep. 2019. PMID: 31776413 Free PMC article.
-
Increasing the Net Negative Charge by Replacement of DOTA Chelator with DOTAGA Improves the Biodistribution of Radiolabeled Second-Generation Synthetic Affibody Molecules.Mol Pharm. 2016 May 2;13(5):1668-78. doi: 10.1021/acs.molpharmaceut.6b00089. Epub 2016 Apr 6. Mol Pharm. 2016. PMID: 27010700
-
Influence of macrocyclic chelators on the targeting properties of (68)Ga-labeled synthetic affibody molecules: comparison with (111)In-labeled counterparts.PLoS One. 2013 Aug 1;8(8):e70028. doi: 10.1371/journal.pone.0070028. Print 2013. PLoS One. 2013. PMID: 23936372 Free PMC article.
-
Comparative evaluation of 111In-labeled NOTA‑conjugated affibody molecules for visualization of HER3 expression in malignant tumors.Oncol Rep. 2015 Aug;34(2):1042-8. doi: 10.3892/or.2015.4046. Epub 2015 Jun 9. Oncol Rep. 2015. PMID: 26059265
Cited by
-
Kinetic analysis of HER2-binding ABY-025 Affibody molecule using dynamic PET in patients with metastatic breast cancer.EJNMMI Res. 2020 Mar 23;10(1):21. doi: 10.1186/s13550-020-0603-9. EJNMMI Res. 2020. PMID: 32201920 Free PMC article.
-
Impact of Radiometal Chelates on In Vivo Visualization of Immune Checkpoint Protein Using Radiolabeled Affibody Molecules.ACS Pharmacol Transl Sci. 2025 Feb 19;8(3):706-717. doi: 10.1021/acsptsci.4c00539. eCollection 2025 Mar 14. ACS Pharmacol Transl Sci. 2025. PMID: 40109742
-
Surface-engineered bacteria in drug development.Microb Biotechnol. 2024 Oct;17(10):e70033. doi: 10.1111/1751-7915.70033. Microb Biotechnol. 2024. PMID: 39403960 Free PMC article. Review.
-
Potential of Nuclear Imaging Techniques to Study the Oral Delivery of Peptides.Pharmaceutics. 2022 Dec 15;14(12):2809. doi: 10.3390/pharmaceutics14122809. Pharmaceutics. 2022. PMID: 36559303 Free PMC article. Review.
-
Molecular Design of HER3-Targeting Affibody Molecules: Influence of Chelator and Presence of HEHEHE-Tag on Biodistribution of 68Ga-Labeled Tracers.Int J Mol Sci. 2019 Mar 2;20(5):1080. doi: 10.3390/ijms20051080. Int J Mol Sci. 2019. PMID: 30832342 Free PMC article.
References
-
- Wang, Z. ErbB Receptors and Cancer. ErbB Receptor Signaling, in Methods In Molecular Biology (ed. Wang, Z.) 3–35 (Humana Press, 2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

