The rice CYP78A gene BSR2 confers resistance to Rhizoctonia solani and affects seed size and growth in Arabidopsis and rice
- PMID: 30679785
- PMCID: PMC6345848
- DOI: 10.1038/s41598-018-37365-1
The rice CYP78A gene BSR2 confers resistance to Rhizoctonia solani and affects seed size and growth in Arabidopsis and rice
Abstract
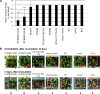
The fungal pathogen Rhizoctonia solani causes devastating diseases in hundreds of plant species. Among these, R. solani causes sheath blight, one of the three major diseases in rice. To date, few genes have been reported that confer resistance to R. solani. Here, rice-FOX Arabidopsis lines identified as having resistance to a bacterial pathogen, Pseudomonas syringae pv. tomato DC3000, and a fungal pathogen, Colletotrichum higginsianum were screened for disease resistance to R. solani. BROAD-SPECTRUM RESISTANCE2 (BSR2), a gene encoding an uncharacterized cytochrome P450 protein belonging to the CYP78A family, conferred resistance to R. solani in Arabidopsis. When overexpressed in rice, BSR2 also conferred resistance to two R. solani anastomosis groups. Both Arabidopsis and rice plants overexpressing BSR2 had slower growth and produced longer seeds than wild-type control plants. In contrast, BSR2-knockdown rice plants were more susceptible to R. solani and displayed faster growth and shorter seeds in comparison with the control. These results indicate that BSR2 is associated with disease resistance, growth rate and seed size in rice and suggest that its function is evolutionarily conserved in both monocot rice and dicot Arabidopsis.
Conflict of interest statement
K.O., M.M.a., H.H. and M.M.o. were funded by Japan Science and Technology Agency. M.M.o. was funded by the Ministry of Agriculture, Forestry, and Fisheries of Japan and has been funded by NARO Bio-oriented Technology Research Advancement Institution. S.M., J.G.D., Y.K., Y.J. and S.S. declare no potential conflict of interest.
Figures






References
-
- Budge GE, Shaw MW, Colyer A, Pietravalle S, Boonham N. Molecular tools to investigate Rhizoctonia solani distribution in soil. Plant Pathol. 2009;58:1071–1080. doi: 10.1111/j.1365-3059.2009.02139.x. - DOI
-
- Yang, G. & Li, C. General Description of Rhizoctonia Species Complex. Plant Pathology 41–52 (2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

