Follicle-Stimulating Hormone Is an Autocrine Regulator of the Ovarian Cancer Metastatic Niche Through Notch Signaling
- PMID: 30680340
- PMCID: PMC6334270
- DOI: 10.1210/js.2018-00272
Follicle-Stimulating Hormone Is an Autocrine Regulator of the Ovarian Cancer Metastatic Niche Through Notch Signaling
Abstract
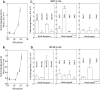
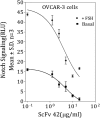
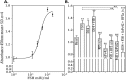
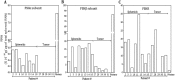
The association between the upregulated Notch and FSH signaling and ovarian cancer is well documented. However, their signaling has been investigated independently and only in the primary tumor tissues. The aim of this study was to investigate the interactive effects of FSH and Notch signaling on ovarian cancer proliferation, formation, and maintenance of disseminated ovarian cancer cells. The roles of Notch and FSH in ovarian cancer pathogenesis were investigated with ovarian cancer cell lines and specific antibodies against Notch and FSH receptor (FSHR). FSH upregulated Notch signaling and proliferation in ovarian cancer cells. High levels of FSH were detected in the ascites of patients with serous ovarian adenocarcinoma. Spheroids from the patients' ascites, as well as the spheroids from ovarian cancer cell lines under low attachment culture conditions, expressed FSHβ subunit mRNA and secreted the hormone into the medium. In contrast, primary ovarian tumor tissues and cell line monolayers expressed very low levels of FSHβ. Ovarian cancer cell spheroids also exhibited higher expression of FSH receptor and Notch downstream genes than their monolayer counterparts. A combination of FSHR and Notch antagonistic antibodies significantly inhibited spheroid formation and cell proliferation in vitro. This study demonstrates that spheroids in ascites express and secrete FSH, which regulates cancer cell proliferation and spheroidogenesis through Notch signaling, suggesting that FSH is an autocrine regulator of cancer metastasis. Furthermore, Notch and FSHR are potential immunotherapeutic targets for ovarian cancer treatment.
Figures







References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Choi J-H, Wong AS, Huang H-F, Leung PC. Gonadotropins and ovarian cancer. Endocr Rev. 2007;28(4):440–461. - PubMed
-
- Gwinn ML, Lee NC, Rhodes PH, Layde PM, Rubin GL. Pregnancy, breast feeding, and oral contraceptives and the risk of epithelial ovarian cancer. J Clin Epidemiol. 1990;43(6):559–568. - PubMed
-
- Havrilesky LJ, Moorman PG, Lowery WJ, Gierisch JM, Coeytaux RR, Urrutia RP, Dinan M, McBroom AJ, Hasselblad V, Sanders GD, Myers ER. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol. 2013;122(1):139–147. - PubMed
-
- Ryle M. Gonadotropins and ovarian function. Acta Eur Fertil. 1973;4(3):113–122. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
