EGFR amplification and classical subtype are associated with a poor response to bevacizumab in recurrent glioblastoma
- PMID: 30680510
- PMCID: PMC6752204
- DOI: 10.1007/s11060-019-03102-5
EGFR amplification and classical subtype are associated with a poor response to bevacizumab in recurrent glioblastoma
Abstract
Purpose: The highly vascular malignant brain tumor glioblastoma (GBM) appears to be an ideal target for anti-angiogenic therapy; however, clinical trials to date suggest the VEGF antibody bevacizumab affects only progression-free survival. Here we analyze a group of patients with GBM who received bevacizumab treatment at recurrence and are stratified according to tumor molecular and genomic profile (TCGA classification), with the goal of identifying molecular predictors of the response to bevacizumab.
Methods: We performed a retrospective review of patients with a diagnosis of glioblastoma who were treated with bevacizumab in the recurrent setting at our hospital, from 2006 to 2014. Treatment was discontinued by the treating neuro-oncologists, based on clinical and radiographic criteria. Pre- and post-treatment imaging and genomic subtype were available on 80 patients. We analyzed time on bevacizumab and time to progression. EGFR gene amplification was determined by FISH.
Results: Patients with classical tumors had a significantly shorter time on bevacizumab than mesenchymal, and proneural patients (2.7 vs. 5.1 vs. 6.4 and 6.0 months respectively, p = 0.011). Classical subtype and EGFR gene amplification were significantly associated with a shorter time to progression both in univariate (p < 0.001 and p = 0.007, respectively) and multivariate analysis (both p = 0.010).
Conclusion: EGFR gene amplification and classical subtype by TCGA analysis are associated with significantly shorter time to progression for patients with recurrent GBM when treated with bevacizumab. These findings can have a significant impact on decision-making and should be further validated prospectively.
Keywords: Bevacizumab; Classical; EGFR; Glioblastoma; Mesenchymal; Proneural.
Conflict of interest statement
Figures
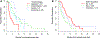

Similar articles
-
Third-line therapy in recurrent glioblastoma: is it another chance for bevacizumab?J Neurooncol. 2018 Sep;139(2):383-388. doi: 10.1007/s11060-018-2873-x. Epub 2018 Apr 18. J Neurooncol. 2018. PMID: 29671196
-
Identification of transcriptome signature for predicting clinical response to bevacizumab in recurrent glioblastoma.Cancer Med. 2018 May;7(5):1774-1783. doi: 10.1002/cam4.1439. Epub 2018 Mar 23. Cancer Med. 2018. PMID: 29573206 Free PMC article.
-
Correlation of radiological and immunochemical parameters with clinical outcome in patients with recurrent glioblastoma treated with Bevacizumab.Clin Transl Oncol. 2019 Oct;21(10):1413-1423. doi: 10.1007/s12094-019-02070-6. Epub 2019 Mar 15. Clin Transl Oncol. 2019. PMID: 30877636
-
The role of bevacizumab in the treatment of glioblastoma.J Neurooncol. 2017 Jul;133(3):455-467. doi: 10.1007/s11060-017-2477-x. Epub 2017 May 19. J Neurooncol. 2017. PMID: 28527008 Review.
-
Use of bevacizumab in recurrent glioblastoma.CNS Oncol. 2015;4(3):157-69. doi: 10.2217/cns.15.8. Epub 2015 Apr 23. CNS Oncol. 2015. PMID: 25906439 Free PMC article. Review.
Cited by
-
Auraptene inhibits migration, invasion and metastatic behavior of human malignant glioblastoma cells: An in vitro and in silico study.Avicenna J Phytomed. 2024 May-Jun;14(3):349-364. doi: 10.22038/AJP.2023.23586. Avicenna J Phytomed. 2024. PMID: 39086858 Free PMC article.
-
Different states of stemness of glioblastoma stem cells sustain glioblastoma subtypes indicating novel clinical biomarkers and high-efficacy customized therapies.J Exp Clin Cancer Res. 2023 Sep 21;42(1):244. doi: 10.1186/s13046-023-02811-0. J Exp Clin Cancer Res. 2023. PMID: 37735434 Free PMC article.
-
Small cell glioblastoma multiforme: a case series and clinicopathological update.CNS Oncol. 2020 Dec 1;9(4):CNS63. doi: 10.2217/cns-2020-0016. Epub 2020 Dec 7. CNS Oncol. 2020. PMID: 33283529 Free PMC article.
-
Genes of the Ubiquitin Proteasome System Qualify as Differential Markers in Malignant Glioma of Astrocytic and Oligodendroglial Origin.Cell Mol Neurobiol. 2023 May;43(4):1425-1452. doi: 10.1007/s10571-022-01261-0. Epub 2022 Jul 27. Cell Mol Neurobiol. 2023. PMID: 35896929 Free PMC article. Review.
-
Update on Chemotherapeutic Approaches and Management of Bevacizumab Usage for Glioblastoma.Pharmaceuticals (Basel). 2020 Dec 16;13(12):470. doi: 10.3390/ph13120470. Pharmaceuticals (Basel). 2020. PMID: 33339404 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

