Porous Silicon Nanoneedles Modulate Endocytosis to Deliver Biological Payloads
- PMID: 30680803
- PMCID: PMC6606440
- DOI: 10.1002/adma.201806788
Porous Silicon Nanoneedles Modulate Endocytosis to Deliver Biological Payloads
Abstract
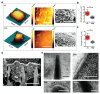
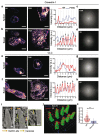
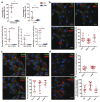
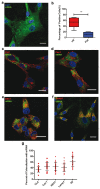
Owing to their ability to efficiently deliver biological cargo and sense the intracellular milieu, vertical arrays of high aspect ratio nanostructures, known as nanoneedles, are being developed as minimally invasive tools for cell manipulation. However, little is known of the mechanisms of cargo transfer across the cell membrane-nanoneedle interface. In particular, the contributions of membrane piercing, modulation of membrane permeability and endocytosis to cargo transfer remain largely unexplored. Here, combining state-of-the-art electron and scanning ion conductance microscopy with molecular biology techniques, it is shown that porous silicon nanoneedle arrays concurrently stimulate independent endocytic pathways which contribute to enhanced biomolecule delivery into human mesenchymal stem cells. Electron microscopy of the cell membrane at nanoneedle sites shows an intact lipid bilayer, accompanied by an accumulation of clathrin-coated pits and caveolae. Nanoneedles enhance the internalization of biomolecular markers of endocytosis, highlighting the concurrent activation of caveolae- and clathrin-mediated endocytosis, alongside macropinocytosis. These events contribute to the nanoneedle-mediated delivery (nanoinjection) of nucleic acids into human stem cells, which distribute across the cytosol and the endolysosomal system. This data extends the understanding of how nanoneedles modulate biological processes to mediate interaction with the intracellular space, providing indications for the rational design of improved cell-manipulation technologies.
Keywords: biointerface; drug delivery; endocytosis; nanoneedles; porous silicon.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF. Nature. 2016;538:183. - PubMed
-
- Chiappini C. ACS Sens. 2017;2:1086. - PubMed
-
- McKnight TE, Melechko AV, Griffin GD, Guillorn MA, Merkulov VI, Serna F, Hensley DK, Doktycz MJ, Lowndes DH, Simpson ML. Nanotechnology. 2003;14:551.
-
- Kim W, Ng JK, Kunitake ME, Conklin BR, Yang P. J Am Chem Soc. 2007;129:7228. - PubMed
MeSH terms
Substances
Grants and funding
- 302638/European Framework Programme 7 Marie Curie Actions
- NC/R001006/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- 2017R1A6A3A03007397/Ministry of Education
- Stoneygate Trust
- BB/D020875/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 098411/Z/12/Z/Wellcome Trust Senior Investigator Award
- 098411/WT_/Wellcome Trust/United Kingdom
- Robert Luff Foundation
- Bloom Foundation
- Rosetrees Trust
- EP/K020641/1/Engineering and Physical Science Research Council
- Department of Bioengineering, Imperial College London
- Imperial College London
- 676137/NanoMed Marie Skłodowska-Curie ITN
- Department of Medicine
- MC_PC_16048/MRC_/Medical Research Council/United Kingdom
- ERC StG 759577/ERC_/European Research Council/International
- 262613/Research Council of Norway
- Royal Society Newton International Fellowship
LinkOut - more resources
Full Text Sources
Other Literature Sources

