Non-alcoholic steatohepatitis aggravates nitric oxide synthase inhibition-induced arteriosclerosis in SHRSP5/Dmcr rat model
- PMID: 30680827
- PMCID: PMC6384504
- DOI: 10.1111/iep.12301
Non-alcoholic steatohepatitis aggravates nitric oxide synthase inhibition-induced arteriosclerosis in SHRSP5/Dmcr rat model
Abstract
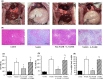
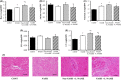
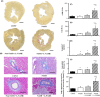
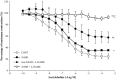
Non-alcoholic steatohepatitis (NASH) is linked to increased cardiovascular risk, independent of the broad spectrum of metabolic syndrome risk factors. Stroke-prone (SP) spontaneously hypertensive rats (SHRSP5/Dmcr) fed a high-fat and high-cholesterol (HFC) diet developed hepatic lesions similar to those in human NASH pathology. These rats simultaneously developed lipid deposits in the mesenteric arteries, cardiac fibrosis, endothelial dysfunction and left ventricle (LV) diastolic dysfunction. However, the intermediary factors between NASH and cardiovascular disease are still unknown. We investigated whether NASH aggravates nitric oxide (NO) synthase inhibition-induced arteriosclerosis in SHRSP5/Dmcr rats. Wistar Kyoto and SHRSP5/Dmcr rats were divided into 4 groups of 5 and fed the stroke-prone (SP) or HFC diets for 8 weeks. To induce NO synthase inhibition, Nω -nitro-L-arginine methyl ester hydrochloride (L-NAME) mixed with drinking water was administered in the final 2 weeks. The NASH+L-NAME group demonstrated the following characteristics related to arteriosclerosis and myocardial ischaemia: (a) LV systolic dysfunction with asynergy, (b) replacement fibrosis caused by the shedding of cardiomyocytes and (c) arterial lipid deposition and coronary occlusion secondary to endothelial dysfunction. These characteristics were not observed in the NASH or non-NASH+L-NAME groups. The SHRSP5/Dmcr rat model demonstrates that NASH significantly aggravates cardiovascular risk.
Keywords: SHRSP5/Dmcr; atherosclerosis; endothelial function; myocardial ischaemia; non-alcoholic steatohepatitis.
© 2019 The Authors. International Journal of Experimental Pathology © 2019 International Journal of Experimental Pathology.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
-
- Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844‐1850. - PubMed
-
- Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258‐267. - PubMed
-
- Sookoian S, Pirola CJ. Non‐alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600‐607. - PubMed
-
- Targher G, Bertolini L, Poli F, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541‐3546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

