Long-term intake of phenolic compounds attenuates age-related cardiac remodeling
- PMID: 30680911
- PMCID: PMC6413651
- DOI: 10.1111/acel.12894
Long-term intake of phenolic compounds attenuates age-related cardiac remodeling
Abstract
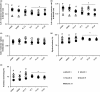
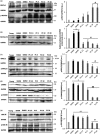
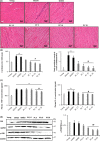
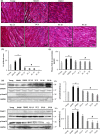
With the onset of advanced age, cardiac-associated pathologies have increased in prevalence. The hallmarks of cardiac aging include cardiomyocyte senescence, fibroblast proliferation, inflammation, and hypertrophy. The imbalance between levels of reactive oxygen species (ROS) and antioxidant enzymes is greatly enhanced in aging cells, promoting cardiac remodeling. In this work, we studied the long-term impact of phenolic compounds (PC) on age-associated cardiac remodeling. Three-month-old Wistar rats were treated for 14 months till middle-age with either 2.5, 5, 10, or 20 mg kg-1 day-1 of PC. PC treatment showed a dose-dependent preservation of cardiac ejection fraction and fractional shortening as well as decreased hypertrophy reflected by left ventricular chamber diameter and posterior wall thickness as compared to untreated middle-aged control animals. Analyses of proteins from cardiac tissue showed that PC attenuated several hypertrophic pathways including calcineurin/nuclear factor of activated T cells (NFATc3), calcium/calmodulin-dependent kinase II (CAMKII), extracellular regulated kinase 1/2 (ERK1/2), and glycogen synthase kinase 3ß (GSK 3ß). PC-treated groups exhibited reduced plasma inflammatory and fibrotic markers and revealed as well ameliorated extracellular matrix remodeling and interstitial inflammation by a downregulated p38 pathway. Myocardia from PC-treated middle-aged rats presented less fibrosis with suppression of profibrotic transforming growth factor-ß1 (TGF-ß1) Smad pathway. Additionally, reduction of apoptosis and oxidative damage in the PC-treated groups was reflected by elevated antioxidant enzymes and reduced RNA/DNA damage markers. Our findings pinpoint that a daily consumption of phenolic compounds could preserve the heart from the detrimental effects of aging storm.
Keywords: aging; cardiac remodeling; fibrosis; hypertrophy; oxidative stress; phenolic compounds.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Metabolic Changes in Cardiac Aging.Rev Cardiovasc Med. 2023 Mar 6;24(3):82. doi: 10.31083/j.rcm2403082. eCollection 2023 Mar. Rev Cardiovasc Med. 2023. PMID: 39077479 Free PMC article. Review.
-
Targeting mitochondrial reactive oxygen species-mediated oxidative stress attenuates nicotine-induced cardiac remodeling and dysfunction.Sci Rep. 2021 Jul 5;11(1):13845. doi: 10.1038/s41598-021-93234-4. Sci Rep. 2021. PMID: 34226619 Free PMC article.
-
Ameliorative Effect of Epigallocatechin Gallate on Cardiac Hypertrophy and Fibrosis in Aged Rats.J Cardiovasc Pharmacol. 2018 Feb;71(2):65-75. doi: 10.1097/FJC.0000000000000545. J Cardiovasc Pharmacol. 2018. PMID: 29419571
-
Dapagliflozin: a sodium-glucose cotransporter 2 inhibitor, attenuates angiotensin II-induced cardiac fibrotic remodeling by regulating TGFβ1/Smad signaling.Cardiovasc Diabetol. 2021 Jun 11;20(1):121. doi: 10.1186/s12933-021-01312-8. Cardiovasc Diabetol. 2021. PMID: 34116674 Free PMC article.
-
Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats.Am J Physiol Heart Circ Physiol. 2007 Oct;293(4):H2093-100. doi: 10.1152/ajpheart.00088.2007. Epub 2007 Jul 13. Am J Physiol Heart Circ Physiol. 2007. PMID: 17630351
Cited by
-
Metabolic Changes in Cardiac Aging.Rev Cardiovasc Med. 2023 Mar 6;24(3):82. doi: 10.31083/j.rcm2403082. eCollection 2023 Mar. Rev Cardiovasc Med. 2023. PMID: 39077479 Free PMC article. Review.
-
Delphinidin attenuates pathological cardiac hypertrophy via the AMPK/NOX/MAPK signaling pathway.Aging (Albany NY). 2020 Mar 25;12(6):5362-5383. doi: 10.18632/aging.102956. Epub 2020 Mar 25. Aging (Albany NY). 2020. PMID: 32209725 Free PMC article.
-
Potential health benefits of phenolic compounds in grape processing by-products.Food Sci Biotechnol. 2019 May 27;28(6):1607-1615. doi: 10.1007/s10068-019-00628-2. eCollection 2019 Dec. Food Sci Biotechnol. 2019. PMID: 31807333 Free PMC article. Review.
-
Innovative Technologies for Extraction and Microencapsulation of Bioactives from Plant-Based Food Waste and their Applications in Functional Food Development.Foods. 2021 Jan 30;10(2):279. doi: 10.3390/foods10020279. Foods. 2021. PMID: 33573135 Free PMC article. Review.
-
Grape Pomace: A Review of Its Bioactive Phenolic Compounds, Health Benefits, and Applications.Molecules. 2025 Jan 17;30(2):362. doi: 10.3390/molecules30020362. Molecules. 2025. PMID: 39860231 Free PMC article. Review.
References
-
- Benjamin, E. J. , Virani, S. S. , Callaway, C. W. , Chamberlain, A. M. , Chang, A. R. , Cheng, S. , & … Muntner P., (2018). Heart disease and stroke statistics-2018 update: A report from the american heart association. Circulation, 137(12), e67–e492. https://doi.org/10.1161/CIR.0000000000000558 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

