Does In Vitro Cytochrome P450 Downregulation Translate to In Vivo Drug-Drug Interactions? Preclinical and Clinical Studies With 13-cis-Retinoic Acid
- PMID: 30681285
- PMCID: PMC6617839
- DOI: 10.1111/cts.12616
Does In Vitro Cytochrome P450 Downregulation Translate to In Vivo Drug-Drug Interactions? Preclinical and Clinical Studies With 13-cis-Retinoic Acid
Abstract
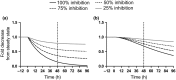
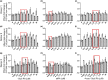
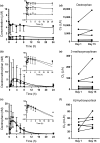
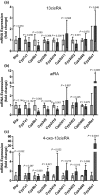
All-trans-retinoic acid (atRA) downregulates cytochrome P450 (CYP)2D6 in several model systems. The aim of this study was to determine whether all active retinoids downregulate CYP2D6 and whether in vitro downregulation translates to in vivo drug-drug interactions (DDIs). The retinoids atRA, 13cisRA, and 4-oxo-13cisRA all decreased CYP2D6 mRNA in human hepatocytes in a concentration-dependent manner. The in vitro data predicted ~ 50% decrease in CYP2D6 activity in humans after dosing with 13cisRA. However, the geometric mean area under plasma concentration-time curve (AUC) ratio for dextromethorphan between treatment and control was 0.822, indicating a weak induction of dextromethorphan clearance following 13cisRA treatment. Similarly, in mice treatment with 4-oxo-13cisRA-induced mRNA expression of multiple mouse Cyp2d genes. In comparison, a weak induction of CYP3A4 in human hepatocytes translated to a weak in vivo induction of CYP3A4. These data suggest that in vitro CYP downregulation may not translate to in vivo DDIs, and better understanding of the mechanisms of CYP downregulation is needed.
© 2019 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
As an Associate Editor for
Figures

References
-
- European Medicines Agency (EMA) . Guideline on the investigation of drug interactions. <https://www.ema.europa.eu/documents/scientific-guideline/guideline-inves...> 2012).
-
- US Food and Drug Administration (FDA) . Clinical Drug Interaction Studies – Study Design, Data Analysis, and Clinical Implications Guidance for Industry. <https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformat...> (2017).
-
- US Food and Drug Administration (FDA) . In Vitro Metabolism‐ and Transporter‐Mediated Drug‐Drug Interaction Studies Guidance for Industry. <https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformat...> (2017).
-
- Intercept Pharmaceuticals Inc. OCALIVA (obeticholic acid) package insert. NDA number 207999. <https://ocalivahcp.com/> (2016).
-
- Hariparsad, N. et al Considerations from the IQ Induction Working Group in Response to Drug‐Drug Interaction Guidance from Regulatory Agencies: focus on downregulation, CYP2C induction, and CYP2B6 positive control. Drug Metab. Dispos. 45, 1049–1059 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

