Pharmacokinetics, Safety, and Tolerability of Single-Dose Intravenous Moxifloxacin in Pediatric Patients: Dose Optimization in a Phase 1 Study
- PMID: 30681729
- PMCID: PMC9252262
- DOI: 10.1002/jcph.1358
Pharmacokinetics, Safety, and Tolerability of Single-Dose Intravenous Moxifloxacin in Pediatric Patients: Dose Optimization in a Phase 1 Study
Abstract
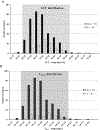
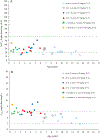
The pharmacokinetics, safety, and tolerability of a single dose of moxifloxacin were characterized in 31 pediatric patients already receiving antibiotics for a suspected or proven infection in an open-label phase 1 study. A dosing strategy for each age cohort (Cohort 1: ≥6 years to ≤14 years; Cohort 2: ≥2 years to <6 years; Cohort 3: >3 month to <2 years) was developed using physiology-based pharmacokinetic modeling combined with a stepwise dosing scheme to obtain a similar exposure to adults receiving 400 mg of moxifloxacin. Doses, adjusted to body weight and age, were gradually escalated from 5 mg/kg in Cohort 1 to 10 mg/kg in Cohort 3 based on interim analysis of the pharmacokinetic and safety data. Plasma and urine samples before and after the 60-minute infusion were collected for the analysis of moxifloxacin and its metabolites using a validated high-pressure liquid chromatography assay with tandem mass spectrometry. Moxifloxacin and metabolite concentrations in plasma were within the ranges observed in adults; however, clearance of all analytes was lower in pediatric patients compared with adults. Population pharmacokinetic analyses using the achieved exposure levels in the 3 age cohorts (with known body weight and clearance) predicted similar efficacy and safety profiles to adults. Moxifloxacin was well tolerated in all pediatric age cohorts. Adverse events related to moxifloxacin were mild or moderate in intensity and showed no correlation with increased weight-adjusted doses. Our findings guided the selection of age-appropriate clinical doses for a subsequent phase 3 clinical trial in pediatric patients with complicated intra-abdominal infections.
Keywords: dose finding; fluoroquinolone; moxifloxacin; pediatrics; pharmacokinetics/pharmacodynamics; phase 1 study.
© 2019, The American College of Clinical Pharmacology.
Conflict of interest statement
Declaration of Conflicting Interests
H.S., S.W., J.L., and K.M.V. are employees of Bayer AG. The Institutions employing J.S.B., J.E.S., L.P.J., and A.C.A. received funds from Bayer, Leverkusen, Germany, to support research staff and clinical costs of the study.
Figures


References
-
- Hackel M, Bhagwat S, Khande H, Joshi P, Patel M, Sahm D. In vitro activity of WCK 771, a new benzoquinolizine quinolone in development, against key bacterial groups from the USA and Europe. Presented at the 55th Interscience Conference of Antimicrobial Agents and Chemotherapy in San Diego, CA, September 17-21, 2015. Poster F-1196.
-
- Hawser S, Hackel M, Bouchillon S, Vente A. Comparative activity of finafloxacin and moxifloxacin against anaerobes. Presented at the 24th European Congress of Clinical Microbiology and Infectious Diseases in Barcelona, Spain, May 10-13, 2014. Poster E-113.
-
- Blondeau JM, Hansen GT. Moxifloxacin: a review of the microbiological, pharmacological, clinical and safety features. Exp Opin Pharmacother. 2001;2:317–335. - PubMed
-
- Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother. 1999;43(suppl B):83–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

