Periarticular Mesenchymal Progenitors Initiate and Contribute to Secondary Ossification Center Formation During Mouse Long Bone Development
- PMID: 30681752
- PMCID: PMC6504254
- DOI: 10.1002/stem.2975
Periarticular Mesenchymal Progenitors Initiate and Contribute to Secondary Ossification Center Formation During Mouse Long Bone Development
Abstract
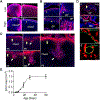
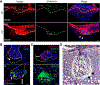
Long bone development involves the embryonic formation of a primary ossification center (POC) in the incipient diaphysis followed by postnatal development of a secondary ossification center (SOC) at each epiphysis. Studies have elucidated major basic mechanisms of POC development, but relatively little is known about SOC development. To gain insights into SOC formation, we used Col2-Cre Rosa-tdTomato (Col2/Tomato) reporter mice and found that their periarticular region contained numerous Tomato-positive lineage cells expressing much higher Tomato fluorescence (termed TomatoH ) than underlying epiphyseal chondrocytes (termed TomatoL ). With time, the TomatoH cells became evident at the SOC invagination site and cartilage canal, increased in number in the expanding SOC, and were present as mesenchymal lineage cells in the subchondral bone. These data were verified in two mouse lineage tracing models, Col2-CreER Rosa-tdTomato and Gli1-CreER Rosa-tdTomato. In vitro tests showed that the periarticular TomatoH cells from Col2/Tomato mice contained mesenchymal progenitors with multidifferentiation abilities. During canal initiation, the cells expressed vascular endothelial growth factor (VEGF) and migrated into epiphyseal cartilage ahead of individual or clusters of endothelial cells, suggesting a unique role in promoting vasculogenesis. Later during SOC expansion, chondrocytes in epiphyseal cartilage expressed VEGF, and angiogenic blood vessels preceded TomatoH cells. Gene expression analyses of microdissected samples revealed upregulation of MMPs in periarticular cells at the invagination site and suggested potential roles for novel kinase and growth factor signaling pathways in regulating SOC canal initiation. In summary, our data indicate that the periarticular region surrounding epiphyseal cartilage contains mesenchymal progenitors that initiate SOC development and form subchondral bone. Stem Cells 2019;37:677-689.
Keywords: Blood vessel; Cartilage; Mesenchymal progenitors; Periarticular layer; Secondary ossification center.
© AlphaMed Press 2019.
Conflict of interest statement
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicated no potential conflicts of interest.
Figures





Similar articles
-
IGF-I Signaling in Osterix-Expressing Cells Regulates Secondary Ossification Center Formation, Growth Plate Maturation, and Metaphyseal Formation During Postnatal Bone Development.J Bone Miner Res. 2015 Dec;30(12):2239-48. doi: 10.1002/jbmr.2563. Epub 2015 Jul 29. J Bone Miner Res. 2015. PMID: 26011431 Free PMC article.
-
The Effects of Indian Hedgehog Deletion on Mesenchyme Cells: Inducing Intermediate Cartilage Scaffold Ossification to Cause Growth Plate and Phalange Joint Absence, Short Limb, and Dwarfish Phenotypes.Stem Cells Dev. 2018 Oct 15;27(20):1412-1425. doi: 10.1089/scd.2018.0071. Epub 2018 Aug 22. Stem Cells Dev. 2018. PMID: 30032718 Free PMC article.
-
Persistent expression of Twist1 in chondrocytes causes growth plate abnormalities and dwarfism in mice.Int J Dev Biol. 2011;55(6):641-7. doi: 10.1387/ijdb.103274rg. Int J Dev Biol. 2011. PMID: 21769775
-
Structure, formation and role of cartilage canals in the developing bone.Ann Anat. 2008;190(4):305-15. doi: 10.1016/j.aanat.2008.02.004. Epub 2008 May 28. Ann Anat. 2008. PMID: 18602255 Review.
-
Recent Insights into Long Bone Development: Central Role of Hedgehog Signaling Pathway in Regulating Growth Plate.Int J Mol Sci. 2019 Nov 20;20(23):5840. doi: 10.3390/ijms20235840. Int J Mol Sci. 2019. PMID: 31757091 Free PMC article. Review.
Cited by
-
Induced regeneration of articular cartilage - identification of a dormant regeneration program for a non-regenerative tissue.Development. 2023 Nov 1;150(21):dev201894. doi: 10.1242/dev.201894. Epub 2023 Nov 8. Development. 2023. PMID: 37882667 Free PMC article.
-
The osteogenic commitment of CD271+CD56+ bone marrow stromal cells (BMSCs) in osteoarthritic femoral head bone.Sci Rep. 2020 Jul 7;10(1):11145. doi: 10.1038/s41598-020-67998-0. Sci Rep. 2020. PMID: 32636407 Free PMC article.
-
Temporal transcriptome features identify early skeletal commitment during human epiphysis development at single-cell resolution.iScience. 2023 Jun 24;26(8):107200. doi: 10.1016/j.isci.2023.107200. eCollection 2023 Aug 18. iScience. 2023. PMID: 37554462 Free PMC article.
-
Stem-Cell-Driven Chondrogenesis: Perspectives on Amnion-Derived Cells.Cells. 2024 Apr 24;13(9):744. doi: 10.3390/cells13090744. Cells. 2024. PMID: 38727280 Free PMC article. Review.
-
The critical role of Hedgehog-responsive mesenchymal progenitors in meniscus development and injury repair.Elife. 2021 Jun 4;10:e62917. doi: 10.7554/eLife.62917. Elife. 2021. PMID: 34085927 Free PMC article.
References
-
- Kronenberg HM Developmental regulation of the growth plate. Nature 2003;423: 332–336. - PubMed
-
- Hall BK, Miyake T All for one and one for all: Condensations and the initiation of skeletal development. Bioessays 2000;22:138–147. - PubMed
-
- Pacifici M, Koyama E, Iwamoto M et al. Development of articular cartilage: What do we know about it and how may it occur? Connect Tissue Res 2000;41:175–184. - PubMed

