Fibrin-Targeted Polymerized Shell Microbubbles as Potential Theranostic Agents for Surgical Adhesions
- PMID: 30681875
- PMCID: PMC6767917
- DOI: 10.1021/acs.langmuir.8b03692
Fibrin-Targeted Polymerized Shell Microbubbles as Potential Theranostic Agents for Surgical Adhesions
Abstract
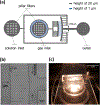
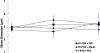
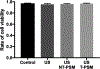
The development of new therapies for surgical adhesions has proven to be difficult as there is no consistently effective way to assess treatment efficacy in clinical trials without performing a second surgery, which can result in additional adhesions. We have developed lipid microbubble formulations that use a short peptide sequence, CREKA, to target fibrin, the molecule that forms nascent adhesions. These targeted polymerized shell microbubbles (PSMs) are designed to allow ultrasound imaging of early adhesions for diagnostic purposes and for evaluating the success of potential treatments in clinical trials while acting as a possible treatment. In this study, we show that CREKA-targeted microbubbles preferentially bind fibrin over fibrinogen and are stable for long periods of time (∼48 h), that these bound microbubbles can be visualized by ultrasound, and that neither these lipid-based bubbles nor their diagnostic-ultrasound-induced vibrations damage mesothelial cells in vitro. Moreover, these bubbles show the potential to identify adhesionlike fibrin formations and may hold promise in blocking or breaking up fibrin formations in vivo.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

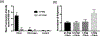
References
-
- Centers for Disease Control. Number of All-Listed Procedures for Discharges from Short-Stay Hospitals, by Procedure Category and Age: United States; 2010.
-
- Schnüriger B; Barmparas G; Branco BC; Lustenberger T; Inaba K; Demetriades D Prevention of Postoperative Peritoneal Adhesions: A Review of the Literature. Am. J. Surg 2011, 201 (1), 111–121. - PubMed
-
- Lyell DJ; Caughey AB; Hu E; Daniels K Peritoneal Closure at Primary Cesarean Delivery and Adhesions. Obstet. Gynecol 2005, 106 (2), 275–280. - PubMed
-
- Duron J-J Postoperative Intraperitoneal Adhesion Pathophysiology. Colorectal Dis. Off. J. Assoc. Coloproctology G. B. Irel 2007, 9 (Suppl 2), 14–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

