Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain
- PMID: 30681985
- PMCID: PMC6424634
- DOI: 10.1097/j.pain.0000000000001458
Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain
Abstract
Chronic pain is associated with neuroplastic changes in the amygdala that may promote hyper-responsiveness to mechanical and thermal stimuli (allodynia and hyperalgesia) and/or enhance emotional and affective consequences of pain. Stress promotes dynorphin-mediated signaling at the kappa opioid receptor (KOR) in the amygdala and mechanical hypersensitivity in rodent models of functional pain. Here, we tested the hypothesis that KOR circuits in the central nucleus of the amygdala (CeA) undergo neuroplasticity in chronic neuropathic pain resulting in increased sensory and affective pain responses. After spinal nerve ligation (SNL) injury in rats, pretreatment with a long-acting KOR antagonist, nor-binaltorphimine (nor-BNI), subcutaneously or through microinjection into the right CeA, prevented conditioned place preference (CPP) to intravenous gabapentin, suggesting that nor-BNI eliminated the aversiveness of ongoing pain. By contrast, systemic or intra-CeA administration of nor-BNI had no effect on tactile allodynia in SNL animals. Using whole-cell patch-clamp electrophysiology, we found that nor-BNI decreased synaptically evoked spiking of CeA neurons in brain slices from SNL but not sham rats. This effect was mediated through increased inhibitory postsynaptic currents, suggesting tonic disinhibition of CeA output neurons due to increased KOR activity as a possible mechanism promoting ongoing aversive aspects of neuropathic pain. Interestingly, this mechanism is not involved in SNL-induced mechanical allodynia. Kappa opioid receptor antagonists may therefore represent novel therapies for neuropathic pain by targeting aversive aspects of ongoing pain while preserving protective functions of acute pain.
Conflict of interest statement
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

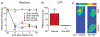


References
-
- Beckerman MA, Van Kempen TA, Justice NJ, Milner TA, Glass MJ. Corticotropin-releasing factor in the mouse central nucleus of the amygdala: ultrastructural distribution in NMDA-NR1 receptor subunit expressing neurons as well as projection neurons to the bed nucleus of the stria terminalis. Exp Neurol 2013;239:120–32. - PMC - PubMed
-
- Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron 2012;73:219–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

