Protein refolding based on high hydrostatic pressure and alkaline pH: Application on a recombinant dengue virus NS1 protein
- PMID: 30682103
- PMCID: PMC6347194
- DOI: 10.1371/journal.pone.0211162
Protein refolding based on high hydrostatic pressure and alkaline pH: Application on a recombinant dengue virus NS1 protein
Abstract
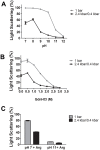
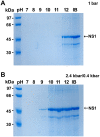
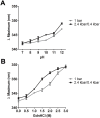
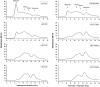
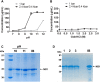
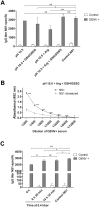
In this study we evaluated the association of high hydrostatic pressure (HHP) and alkaline pH as a minimally denaturing condition for the solubilization of inclusion bodies (IBs) generated by recombinant proteins expressed by Escherichia coli strains. The method was successfully applied to a recombinant form of the dengue virus (DENV) non-structural protein 1 (NS1). The minimal pH for IBs solubilization at 1 bar was 12 while a pH of 10 was sufficient for solubilization at HHP: 2.4 kbar for 90 min and 0.4 kbar for 14 h 30 min. An optimal refolding condition was achieved by compression of IBs at HHP and pH 10.5 in the presence of arginine, oxidized and reduced glutathiones, providing much higher yields (up to 8-fold) than association of HHP and GdnHCl via an established protocol. The refolded NS1, 109 ± 9.5 mg/L bacterial culture was recovered mainly as monomer and dimer, corresponding up to 90% of the total protein and remaining immunologically active. The proposed conditions represent an alternative for the refolding of immunologically active recombinant proteins expressed as IBs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Association of high pressure and alkaline condition for solubilization of inclusion bodies and refolding of the NS1 protein from zika virus.BMC Biotechnol. 2018 Dec 12;18(1):78. doi: 10.1186/s12896-018-0486-2. BMC Biotechnol. 2018. PMID: 30541520 Free PMC article.
-
Refolded dengue virus type 2 NS1 protein expressed in Escherichia coli preserves structural and immunological properties of the native protein.J Virol Methods. 2010 Aug;167(2):186-92. doi: 10.1016/j.jviromet.2010.04.003. Epub 2010 Apr 23. J Virol Methods. 2010. PMID: 20399232
-
Optimization of Dengue-3 recombinant NS1 protein expression in E. coli and in vitro refolding for diagnostic applications.Virus Genes. 2013 Apr;46(2):219-30. doi: 10.1007/s11262-012-0851-5. Epub 2012 Nov 28. Virus Genes. 2013. PMID: 23188193
-
[Research progress in the structure and function of dengue virus non-structural 1 protein].Bing Du Xue Bao. 2014 Nov;30(6):683-8. Bing Du Xue Bao. 2014. PMID: 25868284 Review. Chinese.
-
Plugging the Leak in Dengue Shock.Adv Exp Med Biol. 2018;1062:89-106. doi: 10.1007/978-981-10-8727-1_7. Adv Exp Med Biol. 2018. PMID: 29845527 Review.
Cited by
-
Recombinant PilS: Cloning, Expression and Biochemical Characterization of a Pil-Fimbriae Subunit.Microorganisms. 2022 Jun 7;10(6):1174. doi: 10.3390/microorganisms10061174. Microorganisms. 2022. PMID: 35744689 Free PMC article.
-
Transient Expression of Dengue Virus NS1 Antigen in Nicotiana benthamiana for Use as a Diagnostic Antigen.Front Plant Sci. 2020 Jan 16;10:1674. doi: 10.3389/fpls.2019.01674. eCollection 2019. Front Plant Sci. 2020. PMID: 32010161 Free PMC article.
-
Human growth hormone inclusion bodies present native-like secondary and tertiary structures which can be preserved by mild solubilization for refolding.Microb Cell Fact. 2022 Aug 17;21(1):164. doi: 10.1186/s12934-022-01887-1. Microb Cell Fact. 2022. PMID: 35978337 Free PMC article.
-
Anti-Flavivirus Vaccines: Review of the Present Situation and Perspectives of Subunit Vaccines Produced in Escherichia coli.Vaccines (Basel). 2020 Aug 31;8(3):492. doi: 10.3390/vaccines8030492. Vaccines (Basel). 2020. PMID: 32878023 Free PMC article. Review.
-
Refolding of bioactive human epidermal growth factor from E. coli BL21(DE3) inclusion bodies & evaluations on its in vitro & in vivo bioactivity.Heliyon. 2022 Apr 18;8(4):e09306. doi: 10.1016/j.heliyon.2022.e09306. eCollection 2022 Apr. Heliyon. 2022. PMID: 35497033 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

