Protein refolding based on high hydrostatic pressure and alkaline pH: Application on a recombinant dengue virus NS1 protein
- PMID: 30682103
- PMCID: PMC6347194
- DOI: 10.1371/journal.pone.0211162
Protein refolding based on high hydrostatic pressure and alkaline pH: Application on a recombinant dengue virus NS1 protein
Abstract
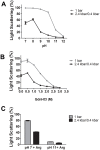
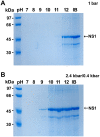
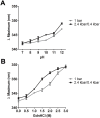
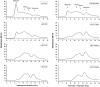
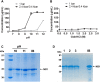
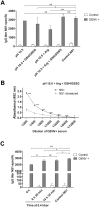
In this study we evaluated the association of high hydrostatic pressure (HHP) and alkaline pH as a minimally denaturing condition for the solubilization of inclusion bodies (IBs) generated by recombinant proteins expressed by Escherichia coli strains. The method was successfully applied to a recombinant form of the dengue virus (DENV) non-structural protein 1 (NS1). The minimal pH for IBs solubilization at 1 bar was 12 while a pH of 10 was sufficient for solubilization at HHP: 2.4 kbar for 90 min and 0.4 kbar for 14 h 30 min. An optimal refolding condition was achieved by compression of IBs at HHP and pH 10.5 in the presence of arginine, oxidized and reduced glutathiones, providing much higher yields (up to 8-fold) than association of HHP and GdnHCl via an established protocol. The refolded NS1, 109 ± 9.5 mg/L bacterial culture was recovered mainly as monomer and dimer, corresponding up to 90% of the total protein and remaining immunologically active. The proposed conditions represent an alternative for the refolding of immunologically active recombinant proteins expressed as IBs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

