Blockade of the PD-1 axis alone is not sufficient to activate HIV-1 virion production from CD4+ T cells of individuals on suppressive ART
- PMID: 30682108
- PMCID: PMC6347234
- DOI: 10.1371/journal.pone.0211112
Blockade of the PD-1 axis alone is not sufficient to activate HIV-1 virion production from CD4+ T cells of individuals on suppressive ART
Abstract
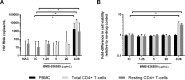
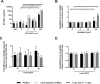
Blockade of the programmed cell death protein/ligand 1 (PD-1/PD-L1) pathway with monoclonal antibodies (mAb) is now commonly used for cancer immunotherapy and has therapeutic potential in chronic viral infections including HIV-1. PD-1/PD-L1 blockade could augment HIV-1-specific immune responses and reverse HIV-1 latency, but the latter effect has not been clearly shown. We tested the ability of the human anti-PD-L1 mAb BMS-936559 and the human anti-PD-1 mAb nivolumab to increase HIV-1 virion production ex vivo from different peripheral blood mononuclear cell populations obtained from donors on suppressive antiretroviral therapy (ART). Fresh peripheral blood mononuclear cells (PBMC), CD8-depleted PBMC, total CD4+ T cells, and resting CD4+ T cells were purified from whole blood of HIV-1-infected donors and cultured in varying concentrations of BMS-936559 (20, 5, or 1.25μg/mL) or nivolumab (5 or 1.25μg/mL), with or without anti-CD3/CD28 stimulatory antibodies. Culture supernatants were assayed for virion HIV-1 RNA by qRT-PCR. Ex vivo exposure to BMS-936559 or nivolumab, with or without anti-CD3/CD28 stimulation, did not consistently increase HIV-1 virion production from blood mononuclear cell populations. Modest (2-fold) increases in virus production were observed in a subset of donors and in some cell types but were not reproducible in longitudinal samples. Cell surface expression of PD-1 and PD-L1 were not associated with changes in virus production. Ex vivo blockade of the PD-1 axis alone has limited effects on HIV-1 latency.
Conflict of interest statement
Research reported in this publication was supported by the University of Pittsburgh, Bristol-Myers Squibb Company, and the Howard Hughes Medical Institute. JWM is a consultant to Gilead Sciences and Merck, and has received grants from Bristol-Myers Squibb Company, Gilead Sciences and Janssen Pharmaceuticals, Inc. He owns stock options in Co-Crystal Pharma, Inc. JB. received support as a research fellow from the Howard Hughes Medical Institute. JC and EF report no competing interests. SC and SWM were employees of Bristol-Myers Squibb (BMS) Company at the time the work was performed. They are no longer affiliated with BMS. This commercial affiliation does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278: 1295–1300. - PubMed
-
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278: 1291–1295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

