Role of aldose reductase in diabetes-induced retinal microglia activation
- PMID: 30682331
- PMCID: PMC6421082
- DOI: 10.1016/j.cbi.2019.01.020
Role of aldose reductase in diabetes-induced retinal microglia activation
Abstract
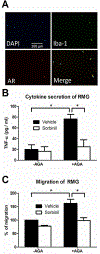
Diabetes-induced hyperglycemia plays a key pathogenic role in degenerative retinal diseases. In diabetic hyperglycemia, aldose reductase (AR) is elevated and linked to the pathogenesis of diabetic retinopathy (DR) and cataract. Retinal microglia (RMG), the resident immune cells in the retina, are thought to contribute to the proinflammatory phenotype in the diabetic eye. However, we have a limited understanding of the potential role of AR expressed in RMG as a mediator of inflammation in the diabetic retina. Glycated proteins accumulate in diabetes, including Amadori-glycated albumin (AGA) which has been shown to induce a proinflammatory phenotype in various tissues. In this study, we investigated the ability of AGA to stimulate inflammatory changes to RMG and macrophages, and whether AR plays a role in this process. In macrophages, treatment with an AR inhibitor (Sorbinil) or genetic knockdown of AR lowered AGA-induced TNF-α secretion (56% and 40%, respectively) as well as cell migration. In a mouse RMG model, AR inhibition attenuated AGA-induced TNF-α secretion and cell migration (67% and 40%, respectively). To further mimic the diabetic milieu in retina, we cultured RMG under conditions of hypoxia and observed the induction of TNF-α and VEGF protein expression. Downregulation of AR in either a pharmacological or genetic manner prevented hypoxia-induced TNF-α and VEGF expression. In our animal study, increased numbers of RMG observed in streptozotocin (STZ)-induced diabetic retina was substantially lower when diabetes was induced in AR knockout mice. Thus, in vitro and in vivo studies demonstrated that AR is involved in diabetes-induced RMG activation, providing a rationale for targeting AR as a therapeutic strategy for DR.
Keywords: Aldose reductase; Amadori-glycated albumin; Hypoxia; Inflammation; Retinal microglia.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Figures



Similar articles
-
Aldose reductase inhibition prevents endotoxin-induced inflammatory responses in retinal microglia.Invest Ophthalmol Vis Sci. 2014 May 2;55(5):2853-61. doi: 10.1167/iovs.13-13487. Invest Ophthalmol Vis Sci. 2014. PMID: 24677107 Free PMC article.
-
Aldose reductase mediates retinal microglia activation.Biochem Biophys Res Commun. 2016 Apr 29;473(2):565-71. doi: 10.1016/j.bbrc.2016.03.122. Epub 2016 Mar 28. Biochem Biophys Res Commun. 2016. PMID: 27033597 Free PMC article.
-
Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes.Diabetes. 2011 Apr;60(4):1122-33. doi: 10.2337/db10-1160. Epub 2011 Feb 11. Diabetes. 2011. PMID: 21317295 Free PMC article.
-
The Metaflammatory and Immunometabolic Role of Macrophages and Microglia in Diabetic Retinopathy.Hum Cell. 2021 Nov;34(6):1617-1628. doi: 10.1007/s13577-021-00580-6. Epub 2021 Jul 29. Hum Cell. 2021. PMID: 34324139 Review.
-
Aldose Reductase as a Key Target in the Prevention and Treatment of Diabetic Retinopathy: A Comprehensive Review.Biomedicines. 2024 Mar 27;12(4):747. doi: 10.3390/biomedicines12040747. Biomedicines. 2024. PMID: 38672103 Free PMC article. Review.
Cited by
-
Efficacy and Safety of Femtosecond Laser-Assisted Cataract Surgery with the Catalys Platform in Patients with Type 2 Diabetes and Cataract: A Prospective Cohort Study.Ophthalmol Ther. 2025 Sep;14(9):2301-2312. doi: 10.1007/s40123-025-01218-8. Epub 2025 Jul 29. Ophthalmol Ther. 2025. PMID: 40731110 Free PMC article.
-
Redefining our vision: an updated guide to the ocular immune system.Nat Rev Immunol. 2024 Dec;24(12):896-911. doi: 10.1038/s41577-024-01064-y. Epub 2024 Aug 30. Nat Rev Immunol. 2024. PMID: 39215057 Review.
-
Müller Cells Harboring Exosomal lncRNA OGRU Modulate Microglia Polarization in Diabetic Retinopathy by Serving as miRNA Sponges.Diabetes. 2024 Nov 1;73(11):1919-1934. doi: 10.2337/db23-1015. Diabetes. 2024. PMID: 39178104 Free PMC article.
-
Updating the Pharmacological Effects of α-Mangostin Compound and Unraveling Its Mechanism of Action: A Computational Study Review.Drug Des Devel Ther. 2024 Oct 24;18:4723-4748. doi: 10.2147/DDDT.S478388. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39469723 Free PMC article. Review.
-
Emerging Roles of Dyslipidemia and Hyperglycemia in Diabetic Retinopathy: Molecular Mechanisms and Clinical Perspectives.Front Endocrinol (Lausanne). 2021 Mar 22;12:620045. doi: 10.3389/fendo.2021.620045. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33828528 Free PMC article. Review.
References
-
- International-Diabetes-Federation, IDF Diabetes Atlas 7th ed., Brussels, Belgium, 2015.
-
- Dewanjee S, Das S, Das AK, Bhattacharjee N, Dihingia A, Dua TK, Kalita J, Manna P, Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets, Eur. J. Pharmacol, 833 (2018) 472–523. - PubMed
-
- Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, Jonas JB, Lamoureux EL, Cheng CY, Klein BEK, Mitchell P, Klein R, Cheung CMG, Wong TY, Incidence and progression of diabetic retinopathy: a systematic review, The lancet. Diabetes & endocrinology, (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

