The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus
- PMID: 30682775
- PMCID: PMC6409565
- DOI: 10.3390/v11020097
The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus
Abstract
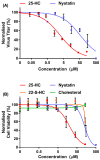
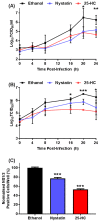
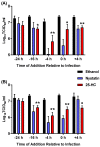
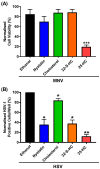
Cholesterol, an essential component of mammalian cells, is also an important factor in the replicative-cycles of several human and animal viruses. The oxysterol, 25-hydroxycholesterol, is produced from cholesterol by the enzyme, cholesterol 25-hydroxylase. 25-hydroxycholesterol (25-HC) has been shown to have anti-viral activities against a wide range of viruses, including a range of positive-sense RNA viruses. In this study, we have investigated the role of 25-HC in norovirus replication using murine norovirus (MNV) as a model system. As a control, we employed herpes simplex virus-1 (HSV-1), a pathogen previously shown to be inhibited by 25-HC. Consistent with previous studies, 25-HC inhibited HSV-1 replication in the MNV-susceptible cell line, RAW264.7. Treating RAW264.7 cells with sub-cytotoxic concentrations of 25-HC reduced the MNV titers. However, other sterols such as cholesterol or the oxysterol, 22-S-hydroxycholesterol (22-S-HC), did not inhibit MNV replication. Moreover, treating MNV-infected RAW264.7 cells with 25-HC-stimulated caspase 3/7 activity, which leads to enhanced apoptosis and increased cell death. Our study adds noroviruses to the list of viruses inhibited by 25-HC and begins to offer insights into the mechanism behind this inhibition.
Keywords: 25-HC; Apoptosis; MNV; Murine norovirus; Nystatin; Replication.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

References
-
- Green K. Fields Virology. 6th ed. Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia, PA, USA: 2013. Chapter 20—Caliciviridae: The noroviruses.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

