Preparation and Utility of N-Alkynyl Azoles in Synthesis
- PMID: 30682796
- PMCID: PMC6384649
- DOI: 10.3390/molecules24030422
Preparation and Utility of N-Alkynyl Azoles in Synthesis
Abstract
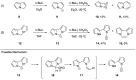
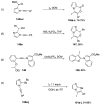
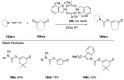
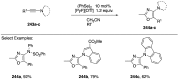
Heteroatom-substituted alkynes have attracted a significant amount of interest in the synthetic community due to the polarized nature of these alkynes and their utility in a wide range of reactions. One specific class of heteroatom-substituted alkynes combines this utility with the presence of an azole moiety. These N-alkynyl azoles have been known for nearly 50 years, but recently there has been a tremendous increase in the number of reports detailing the synthesis and utility of this class of compound. While much of the chemistry of N-alkynyl azoles mirrors that of the more extensively studied N-alkynyl amides (ynamides), there are notable exceptions. In addition, as azoles are extremely common in natural products and pharmaceuticals, these N-alkynyl azoles have high potential for accessing biologically important compounds. In this review, the literature reports of N-alkynyl azole synthesis, reactions, and uses have been assembled. Collectively, these reports demonstrate the growth in this area and the promise of exploiting N-alkynyl azoles in synthesis.
Keywords: alkynes; carbenes; cyclizations; natural products; polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









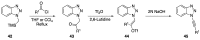
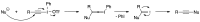



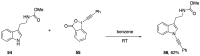

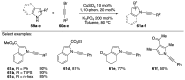







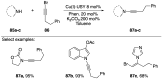






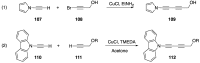



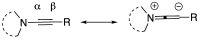






















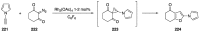


















Similar articles
-
Ruthenium-catalyzed [2 + 2] cycloadditions of bicyclic alkenes with alkynyl phosphonates.J Org Chem. 2009 Aug 7;74(15):5762-5. doi: 10.1021/jo9010206. J Org Chem. 2009. PMID: 19572573
-
Gold-catalyzed cyclizations of alkynol-based compounds: synthesis of natural products and derivatives.Molecules. 2011 Sep 13;16(9):7815-43. doi: 10.3390/molecules16097815. Molecules. 2011. PMID: 22143545 Free PMC article. Review.
-
A concise synthesis of (alkynyl)(trifluoromethyl)sulfanes via a bismuth(III)-promoted reaction of trimethyl(alkynyl)silane with trifluoromethanesulfanylamide.Org Biomol Chem. 2014 Oct 14;12(38):7629-33. doi: 10.1039/c4ob01451k. Org Biomol Chem. 2014. PMID: 25141967
-
Synthesis of highly substituted 3-formylfurans by a gold(I)-catalyzed oxidation/1,2-alkynyl migration/cyclization cascade.Angew Chem Int Ed Engl. 2014 Apr 1;53(14):3715-9. doi: 10.1002/anie.201310146. Epub 2014 Feb 24. Angew Chem Int Ed Engl. 2014. PMID: 24616030
-
Cross-Dehydrogenative Coupling Reactions Between C(sp)-H and X-H (X = N, P, S, Si, Sn) Bonds: An Environmentally Benign Access to Heteroatom-Substituted Alkynes.Top Curr Chem (Cham). 2019 Jul 4;377(4):20. doi: 10.1007/s41061-019-0245-4. Top Curr Chem (Cham). 2019. PMID: 31273478 Review.
Cited by
-
Asymmetric synthesis with ynamides: unique reaction control, chemical diversity and applications.Chem Soc Rev. 2020 Dec 7;49(23):8543-8583. doi: 10.1039/d0cs00769b. Epub 2020 Oct 19. Chem Soc Rev. 2020. PMID: 33073285 Free PMC article.
References
-
- Ficini J. Ynamine—Versatile Tool in Organic-Synthesis. Tetrahedron. 1976;32:1449–1486. doi: 10.1016/0040-4020(76)85200-3. - DOI
-
- Zificsak C.A., Mulder J.A., Hsung R.P., Rameshkumar C., Wei L.L. Recent advances in the chemistry of ynamines and ynamides. Tetrahedron. 2001;57:7575–7606. doi: 10.1016/S0040-4020(01)00681-0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

