Multidisciplinary intervention to improve medication safety in nursing home residents: protocol of a cluster randomised controlled trial (HIOPP-3-iTBX study)
- PMID: 30683060
- PMCID: PMC6347799
- DOI: 10.1186/s12877-019-1027-0
Multidisciplinary intervention to improve medication safety in nursing home residents: protocol of a cluster randomised controlled trial (HIOPP-3-iTBX study)
Abstract
Background: Medication safety is an important health issue for nursing home residents (NHR). They usually experience polypharmacy and often take potentially inappropriate medications (PIM) and antipsychotics. This, coupled with a frail health state, makes NHR particularly vulnerable to adverse drug events (ADE). The value of systematic medication reviews and interprofessional co-operation for improving medication quality in NHR has been recognized. Yet the evidence of a positive effect on NHR' health and wellbeing is inconclusive at this stage. This study investigates the effects of pharmacists' medication reviews linked with measures to strengthen interprofessional co-operation on NHR' medication quality, health status and health care use.
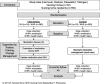
Methods: Pragmatic cluster randomised controlled trial in nursing homes in four regions of Germany. A total of 760 NHR will be recruited. Inclusion: NHR aged 65 years and over with an estimated life expectancy of at least six months. Intervention with four elements: i) introduction of a pharmacist's medication review combined with a communication pathway to the prescribing general practitioners (GPs) and nursing home staff, ii) facilitation of change in the interprofessional cooperation, iii) educational training and iv) a "toolbox" to facilitate implementation in daily practice.
Analysis: primary outcome - proportion of residents receiving PIM and ≥ 2 antipsychotics at six months follow-up. Secondary outcomes - cognitive function, falls, quality of life, medical emergency contacts, hospital admissions, and health care costs.
Discussion: The trial assesses the effects of a structured interprofessional medication management for NHR in Germany. It follows the participatory action research approach and closely involves the three professional groups (nursing staff, GPs, pharmacists) engaged in the medication management. A handbook based on the experiences of the trial in nursing homes will be produced for a rollout into routine practice in Germany.
Trial registration: Registered in the German register of clinical studies (DRKS, study ID DRKS00013588 , primary register) and in the WHO International Clinical Trials Registry Platform (secondary register), both on 25th January 2018.
Keywords: Change management; General practitioners; Interprofessional; Medication review; Medication therapy management; Nursing homes; Nursing staff; Pharmacists; Polypharmacy.
Conflict of interest statement
Ethics approval and consent to participate
Research staff provides eligible participants (nursing home residents and/or their legal representatives) with an information sheet. All participants will sign an informed consent according to the Declaration of Helsinki prior to data collection. Before conducting the interview, the researcher will explain the purpose and duration of the study to the participants. The study was approved by the ethics committee of the Hannover Medical School on 4th of December 2017 (Approval Number: 7655).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Fleischmann N, Tetzlaff B, Werle J, Geister C, Scherer M, Weyerer S, et al. Interprofessional collaboration in nursing homes (interprof): a grounded theory study of general practitioner experiences and strategies to perform nursing home visits. BMC Fam Pract. 2016;17(1):123. doi: 10.1186/s12875-016-0522-z. - DOI - PMC - PubMed
-
- Mc Namara KP, Breken BD, Alzubaidi HT, Bell JS, Dunbar JA, Walker C, et al. Health professional perspectives on the management of multimorbidity and polypharmacy for older patients in Australia. Age Ageing. 2017;46(2):291–299. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical