Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats
- PMID: 30683110
- PMCID: PMC6346555
- DOI: 10.1186/s12951-019-0451-9
Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats
Abstract
Background: Cyclosporin A (CsA) is a promising therapeutic drug for myocardial ischemia reperfusion injury (MI/RI) because of its definite inhibition to the opening of mitochondrial permeability transition pore (mPTP). However, the application of cyclosporin A to treat MI/RI is limited due to its immunosuppressive effect to other normal organ and tissues. SS31 represents a novel mitochondria-targeted peptide which can guide drug to accumulate into mitochondria. In this paper, mitochondria-targeted nanoparticles (CsA@PLGA-PEG-SS31) were prepared to precisely deliver cyclosporin A into mitochondria of ischemic cardiomyocytes to treat MI/RI.
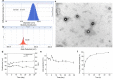
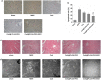
Results: CsA@PLGA-PEG-SS31 was prepared by nanoprecipitation. CsA@PLGA-PEG-SS31 showed small particle size (~ 50 nm) and positive charge due to the modification of SS31 on the surface of nanoparticles. CsA@PLGA-PEG-SS31 was stable for more than 30 days and displayed a biphasic drug release pattern. The in vitro results showed that the intracellular uptake of CsA@PLGA-PEG-SS31 was significantly enhanced in hypoxia reoxygenation (H/R) injured H9c2 cells. CsA@PLGA-PEG-SS31 delivered CsA into mitochondria of H/R injured H9c2 cells and subsequently increased the viability of H/R injured H9c2 cell through inhibiting the opening of mPTP and production of reactive oxygen species. In vivo results showed that CsA@PLGA-PEG-SS31 accumulated in ischemic myocardium of MI/RI rat heart. Apoptosis of cardiomyocyte was alleviated in MI/RI rats treated with CsA@PLGA-PEG-SS31, which resulted in the myocardial salvage and improvement of cardiac function. Besides, CsA@PLGA-PEG-SS31 protected myocardium from damage by reducing the recruitment of inflammatory cells and maintaining the integrity of mitochondrial function in MI/RI rats.
Conclusion: CsA@PLGA-PEG-SS31 exhibited significant cardioprotective effects against MI/RI in rats hearts through protecting mitochondrial integrity, decreasing apoptosis of cardiomyocytes and myocardial infract area. Thus, CsA@PLGA-PEG-SS31 offered a promising therapeutic method for patients with acute myocardial infarction.
Keywords: Cyclosporin A; Mitochondria-targeted peptide; Mitochondrial permeability transition pore; Mitochondrial targeting; Myocardial ischemia/reperfusion injury.
Figures







References
-
- Silva FSG, Costa CF, Marques RJ, Oliveira PJ, Pereira GC. Pharmacological targeting of the mitochondrial permeability transition pore for cardioprotection. Berlin: Springer; 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

