Efficacy of motivating short interventions for smokers in primary care (COSMOS trial): study protocol for a cluster-RCT
- PMID: 30683155
- PMCID: PMC6347802
- DOI: 10.1186/s13063-018-3071-z
Efficacy of motivating short interventions for smokers in primary care (COSMOS trial): study protocol for a cluster-RCT
Abstract
Background: Tobacco abuse is a frequent issue in general practitioners' (GPs') offices, with doctors playing a key role in promoting smoking cessation to their patients. However, not all smokers are ready and willing to give up smoking. Thus, a GP focusing on smoking cessation alone might waste the opportunity to improve his patient's health by supporting a change in another harmful behaviour pattern. The aim of this study is to determine whether multi-thematic coaching will lead to higher overall health benefits without resulting in a reduced rate of successful smoking cessations, compared with a monothematic smoking cessation approach.
Methods: The study is designed as a two-armed, double-blinded, cluster-randomised trial. GPs will be randomly assigned to the intervention or control group. In the intervention group, GPs will undergo training in patient-centred coaching, shared decision-making and motivational interviewing. The control group will be trained in a state-of-the-art smoking cessation algorithm. GPs will approach adult cigarette-smoking patients and advise those included according to the GP's group affiliation. The primary outcome is the between-group difference in the proportion of participants who achieve a beneficial change in at least one of seven different health-related behavioural dimensions, 12 months post baseline. Secondary outcomes include smoking cessation rates and the patients' self-perceived smoking-related motivation, self-efficacy and planning behaviour. Additionally, covariates describing both GPs and patients will be collected before the start of the intervention, and process outcome measures in compliance with the RE-AIM (Reach Effectiveness Adoption Implementation Maintenance) framework will be recorded during the ongoing study.
Discussion: Tobacco consumption is still highly prevalent in the general population and often goes hand in hand with other behaviour patterns with adverse health effects. This study will add to the literature regarding effective strategies available to GPs to address unhealthy behaviour among their smoking patients beyond mere smoking cessation counselling. The study will also establish a basis for decisions about further promotion and dissemination of the coaching under study.
Trial registration: ISRCTN, ISRCTN38129107 . Registered on 2 October 2017.
Keywords: Counselling; Health behaviour; Health promotion; Motivational interviewing; Patient-centredness; Primary care; Shared decision-making; Smoking cessation.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol has been approved by the Ethics Committee of the Canton of Zurich (KEK-ZH Ref no. 2017–02043, 23 January 2018). Informed consent will be sought from all participating GPs and patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
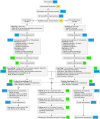
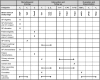
References
-
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53(9):1217–1222. - PubMed
-
- Frei von Tabak—ärztliche Beratung zum Rauchstopp. https://www.freivontabak.ch. Accessed 3 Oct 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

