Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis
- PMID: 30683238
- PMCID: PMC6366854
- DOI: 10.1016/S2214-109X(18)30458-3
Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis
Erratum in
-
Correction to Lancet Glob Health 2019; 7: e191-99.Lancet Glob Health. 2019 Apr;7(4):e419. doi: 10.1016/S2214-109X(19)30046-4. Epub 2019 Feb 19. Lancet Glob Health. 2019. PMID: 30796002 Free PMC article. No abstract available.
Abstract
Background: Xpert MTB/RIF, the most widely used automated nucleic acid amplification test for tuberculosis, is available in more than 130 countries. Although diagnostic accuracy is well documented, anticipated improvements in patient outcomes have not been clearly identified. We performed an individual patient data meta-analysis to examine improvements in patient outcomes associated with Xpert MTB/RIF.
Methods: We searched PubMed, Embase, ClinicalTrials.gov, and the Pan African Clinical Trials Registry from inception to Feb 1, 2018, for randomised controlled trials (RCTs) comparing the use of Xpert MTB/RIF with sputum smear microscopy as tests for tuberculosis diagnosis in adults (aged 18 years or older). We excluded studies of patients with extrapulmonary tuberculosis, and studies in which mortality was not assessed. We used a two-stage approach for our primary analysis and a one-stage approach for the sensitivity analysis. To assess the primary outcome of cumulative 6-month all-cause mortality, we first performed logistic regression models (random effects for cluster randomised trials, with robust SEs for multicentre studies) for each trial, and then pooled the odds ratio (OR) estimates by a fixed-effects (inverse variance) or random-effects (Der Simonian Laird) meta-analysis. We adjusted for age and gender, and stratified by HIV status and previous tuberculosis-treatment history. The study protocol has been registered with PROSPERO, number CRD42014013394.
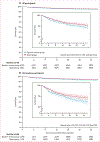
Findings: Our search identified 387 studies, of which five RCTs were eligible for analysis. 8567 adult clinic attendees (4490 [63·5%] of 7074 participants for whom data were available were HIV-positive) were tested for tuberculosis with Xpert MTB/RIF (Xpert group) versus sputum smear microscopy (sputum smear group), across five low-income and middle-income countries (South Africa, Brazil, Zimbabwe, Zambia, and Tanzania). The primary outcome (reported in three studies) occurred in 182 (4·5%) of 4050 patients in the Xpert group and 217 (5·3%) of 4093 patients in the smear group (pooled adjusted OR 0·88, 95% CI 0·68-1·14 [p=0·34]; for HIV-positive individuals OR 0·83, 0·65-1·05 [p=0·12]). Kaplan-Meier estimates showed a lower rate of death (12·73 per 100 person-years in the Xpert group vs 16·38 per 100 person-years in the sputum smear group) for HIV-positive patients (hazard ratio 0·76, 95% CI 0·60-0·97; p=0·03). The risk of bias was assessed as reasonable and the statistical heterogeneity across studies was low (I2<20% for the primary outcome).
Interpretation: Despite individual patient data analysis from five RCTs, we were unable to confidently rule in nor rule out an Xpert MTB/RIF-associated reduction in mortality among outpatients tested for tuberculosis. Reduction in mortality among HIV-positive patients in a secondary analysis suggests the possibility of population-level impact.
Funding: US National Institutes of Health.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests
All other authors declare no competing interests.
Figures
Comment in
-
The impact of Xpert MTB/RIF-do we have a final answer?Lancet Glob Health. 2019 Feb;7(2):e161-e162. doi: 10.1016/S2214-109X(18)30493-5. Lancet Glob Health. 2019. PMID: 30683224 No abstract available.
References
-
- WHO. Global tuberculosis report 2018 2018. http://www.who.int/tb/publications/global_report/en/ (accessed on Dec 1, 2018).
-
- WHO. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Policy statement Geneva: World Health Organization, 2011. - PubMed